covalent bonding
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
what types of atoms bond covalently?
non metal + non metal
how are electrons distributed to give all the atoms a full outer shell?
since all atoms bonded covalently are non metals, they need to gain electrons to have a full outer shell. they share pairs of electrons to do this.
are atoms bonded covalently charged?
no - since the electrons are shared instead of transferred, no atom ends up positively or negatively charged.
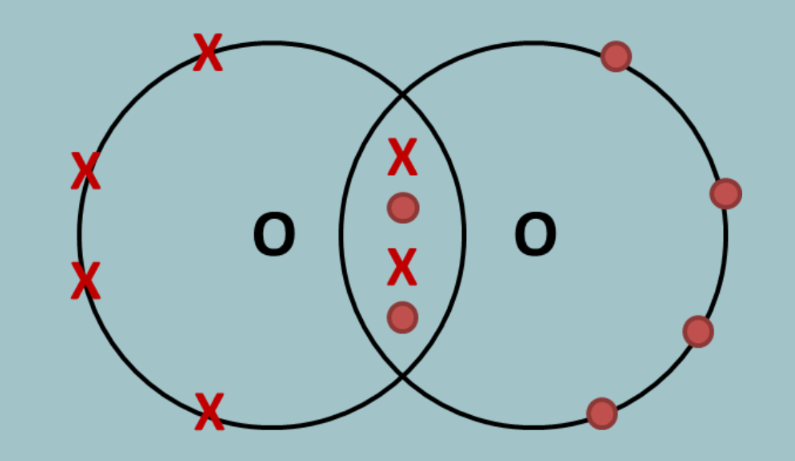
what would the covalent bond for oxygen (dot and cross diagram) look like?
each oxygen atom has 6 electrons in its outer shell, so each need 2 more for a full one. they each share two electrons with each other so that in total, 4 electrons are shared and they now both have a full outer shell.
how strong are the intramolecular covalent bonds?
very strong