VSEPR Geometries & Bond Angles (Vocabulary)
1/17
Earn XP
Description and Tags
Vocabulary flashcards covering VSEPR geometries, electron regions, bond angles, examples, and memorization cues.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
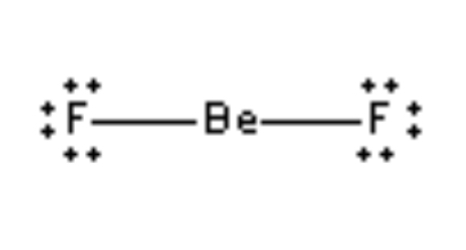
Linear 180°
2 electron regions , 2 bonds, 0 lone pairs
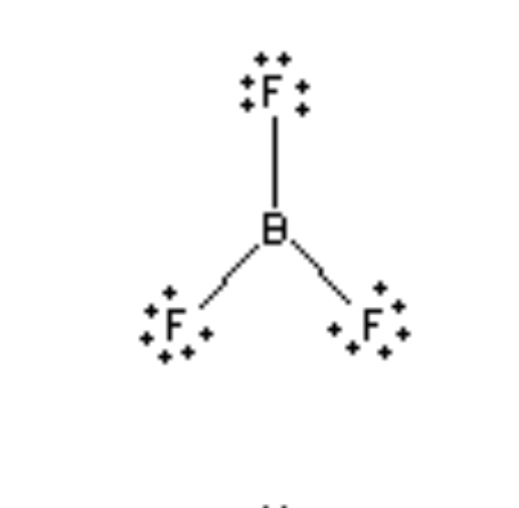
Trigonal planar 120°
3 electron regions, 3 bonds, 0 Lone pairs
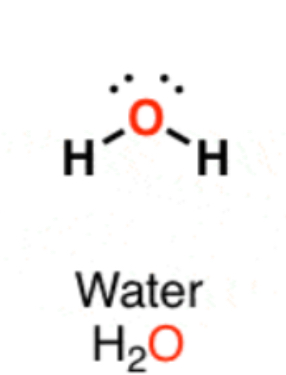
Bent 120°
3 Electron Regions, 2 bonds, 1 lone pair
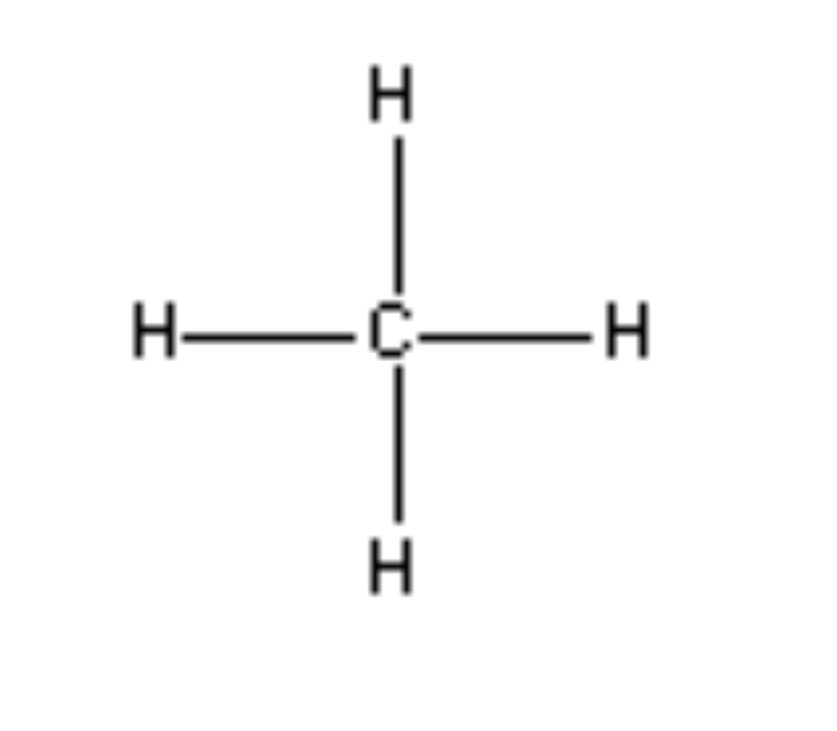
Tetrahedral 109.5°
4 Electron Regions, 4 bonds 0 lone pairs
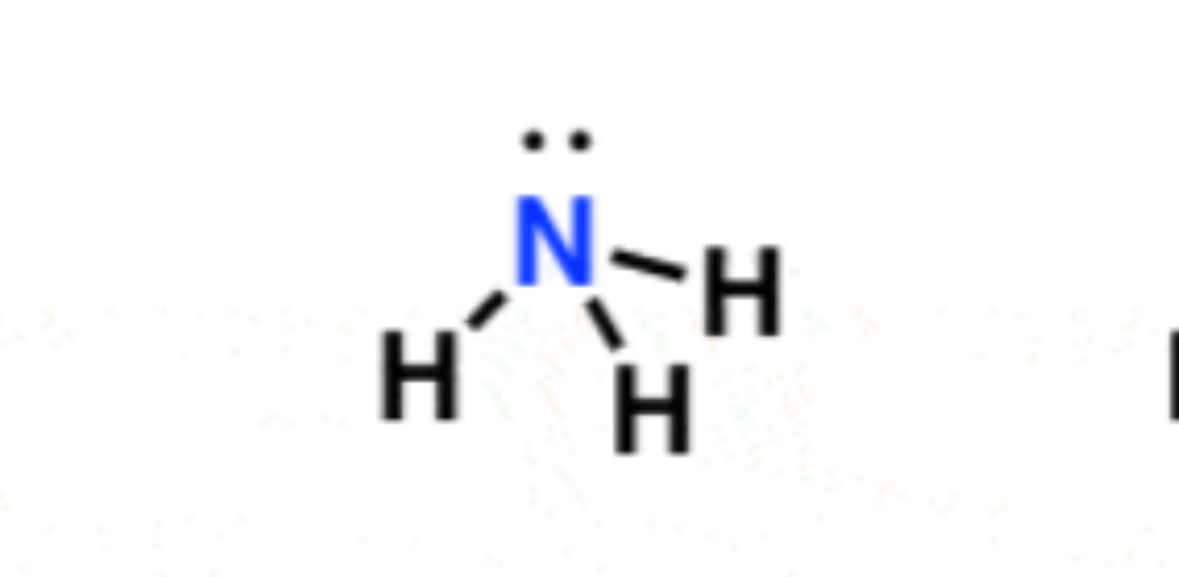
Trigonal pyramidal 107°
4 electrons, 3 bonds, 1 lone pairs
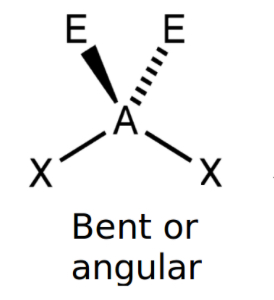
Bent 104.5° (with 4 electrons)
2 bonds, 2 Lone pairs
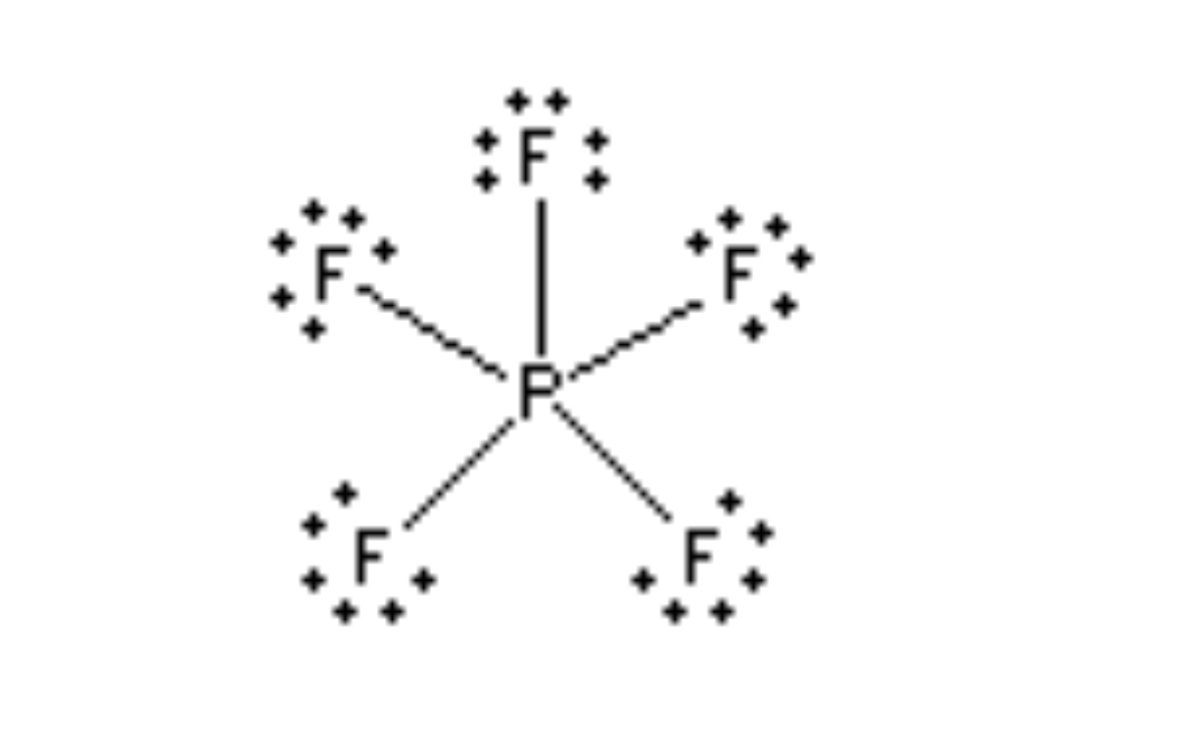
Trigonal bipyramidal 90° (axial), 120° (equatorial)
5 electrons, 5 bonds, 0 lone pairs
See-saw <90°, <120°, <180°
5 electrons, 4 bonds. 1 lone pair
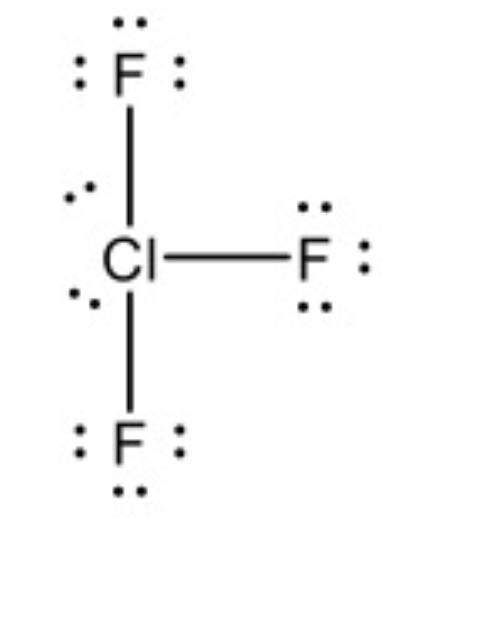
T-shaped 90°
5 Electron regions , 3 bonds, 2 lone pairs
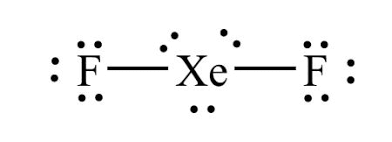
Linear ( 5 electrons) 180°
5 Electron Regions, 2 bonds, 3 lone pairs
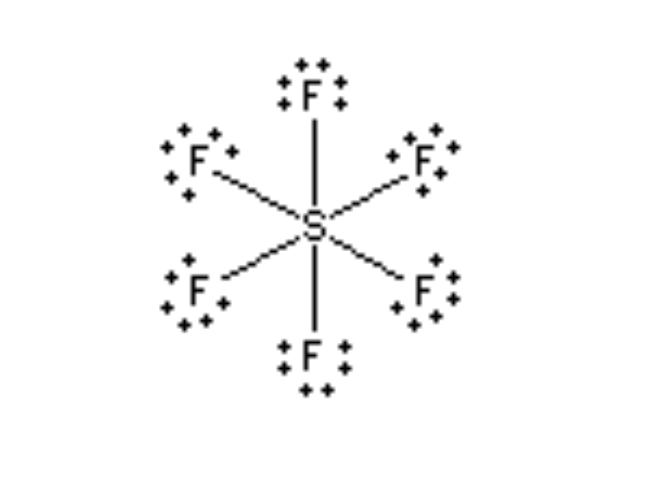
Octahedral 90°
6 Electron regions, 6 bonds. 0 lone pairs
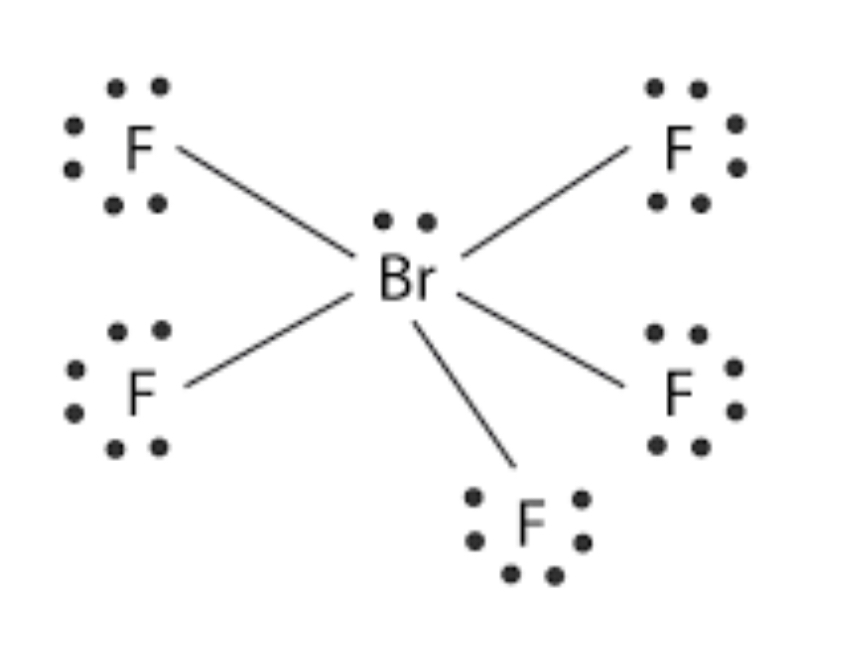
Square pyramidal <90°
6 Electron Regions, 5 bonds, 1 lone pair
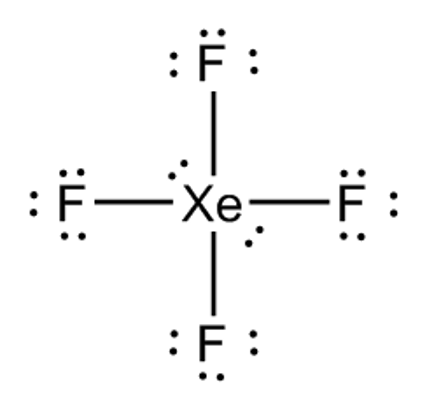
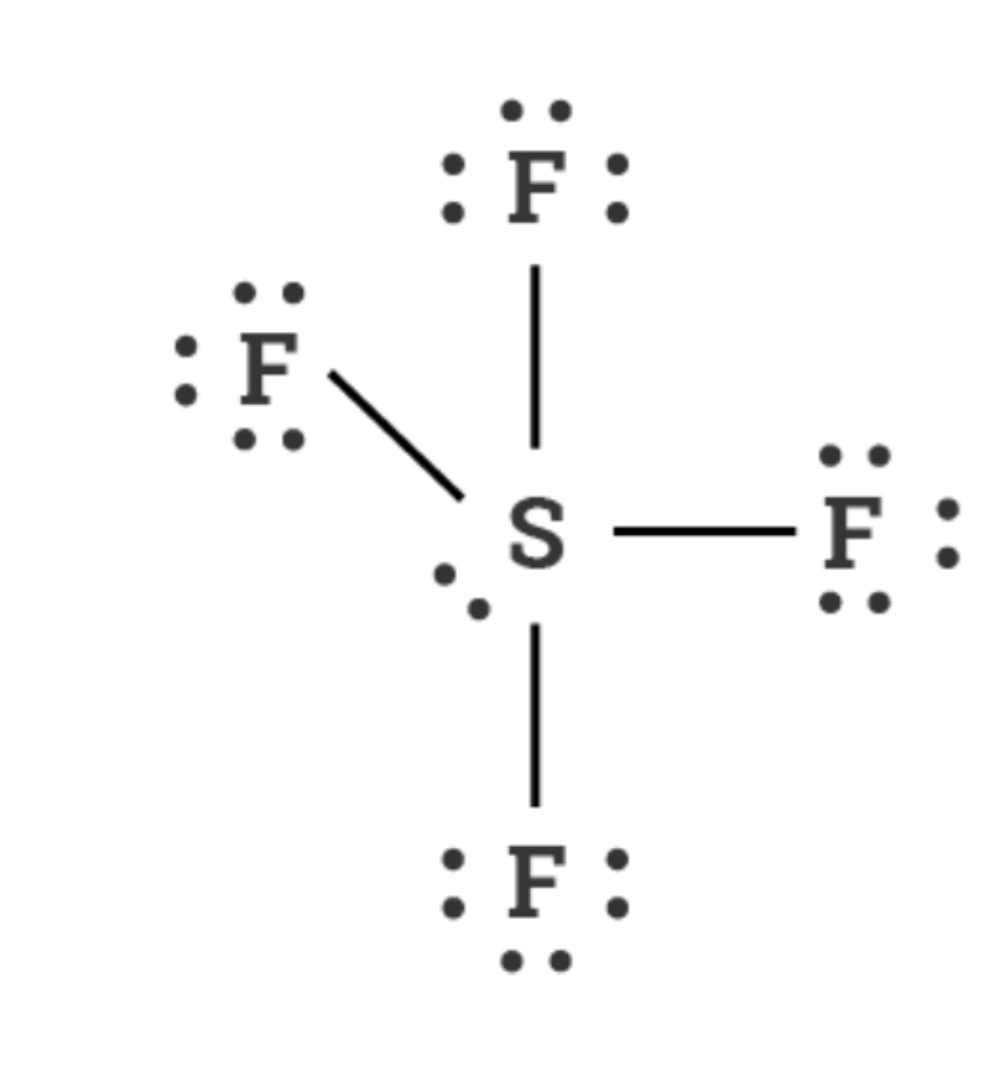
Square planar 90°
6 electrons, 4 bonds, 2 lone pairs
180° (Key angle)
Linear geometry.
120° (Key angle)
Key angle associated with Trigonal planar geometry.
109.5° (Key angle)
Key angle associated with Tetrahedral geometry.
90°/120° mix (Key angle)
Angles characteristic of Trigonal bipyramidal geometry.
90° (Key angle)
Key angle associated with Octahedral geometry.