12. Generating T cells
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
How are unactivated (“naïve”) B and T cells generated against any possible antigen?
Through VDJ recombination, which creates immense diversity of BCRs and TCRs, allowing recognition of virtually any antigen before activation.
Where do naïve T cells come from before they’re activated in lymph nodes?
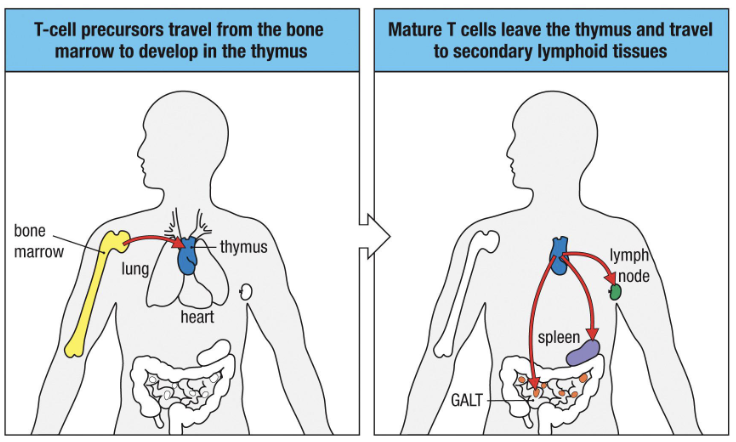
Origin: Precursors from bone marrow → develop in thymus.
Before activation: Enter lymph nodes and wait for dendritic cell presentation.
After activation: Mature → move to peripheral tissues to perform effector functions.
Outline the developmental pathway of T cells.
Hematopoietic stem cells → T cell precursors (bone marrow)
Precursors travel to the thymus (near the heart)
Develop into naïve T cells in the thymus
Naïve T cells exit → secondary lymphoid tissues
“T” stands for “thymus.”
What key processes occur in the thymus during T cell development?
VDJ recombination forms a functional TCR
Cells differentiate into αβ or γδ T cells
Cells further specialize into CD4+ helper or CD8+ cytotoxic T cells
What risk comes with VDJ recombination?
It can generate TCRs or BCRs that recognize self-antigens, causing autoimmune diseases (e.g., type 1 diabetes, lupus).
What four traits make a functional, non-self-reactive T cell?
Functional TCR to detect and respond to antigens
Can bind MHC molecules
Does not bind self-proteins presented on MHC
Is either CD4+ or CD8+
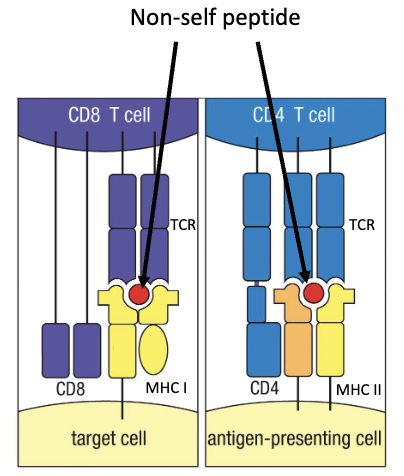
What is the goal of thymic T cell development?
To pass all 4 checkpoints:
✅ Has TCR
✅ Binds MHC
✅ Doesn’t bind to self-proteins
✅ Becomes single positive (CD4 or CD8)
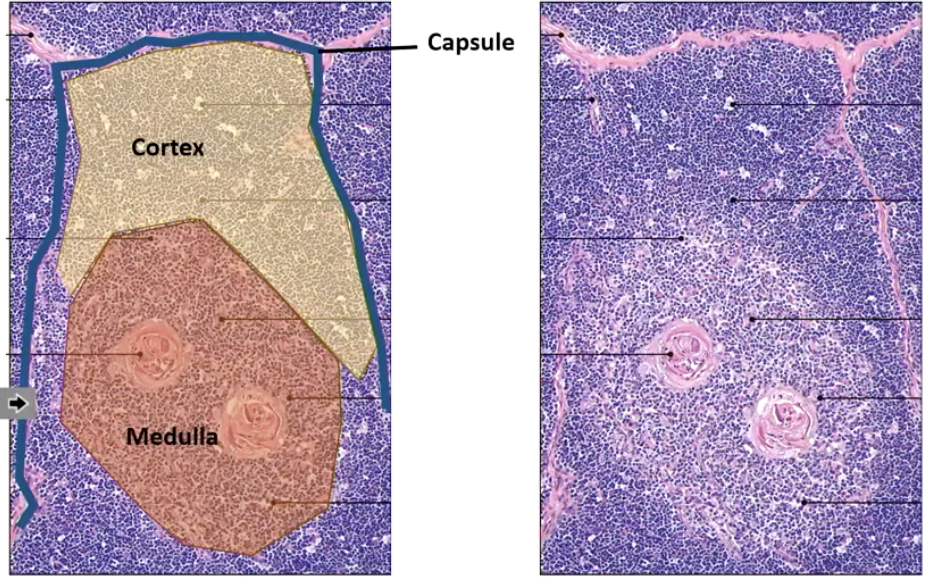
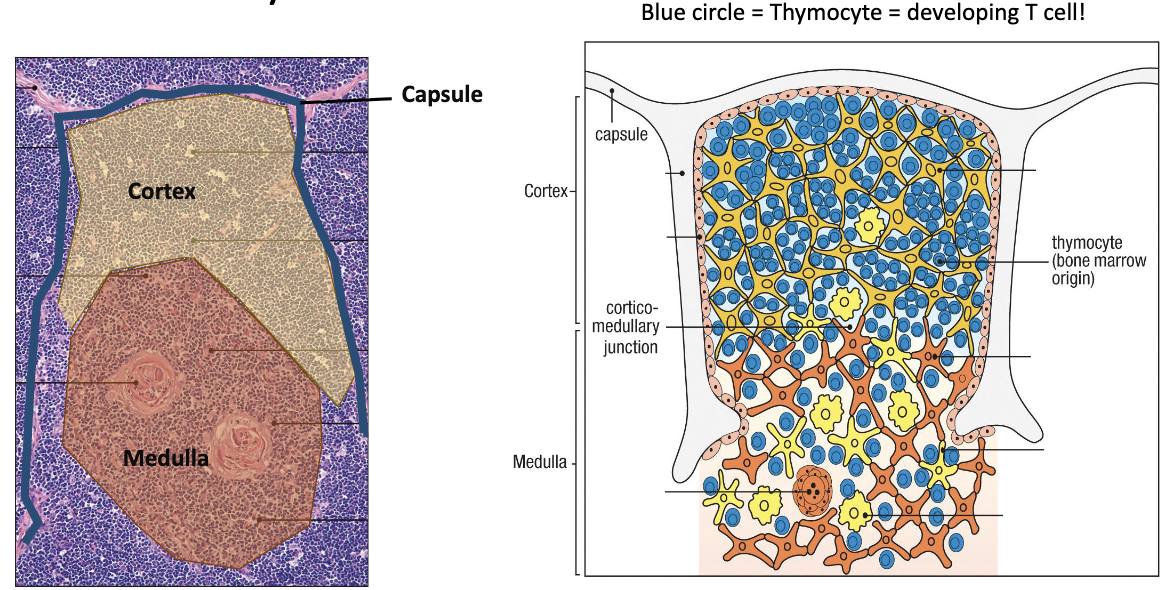
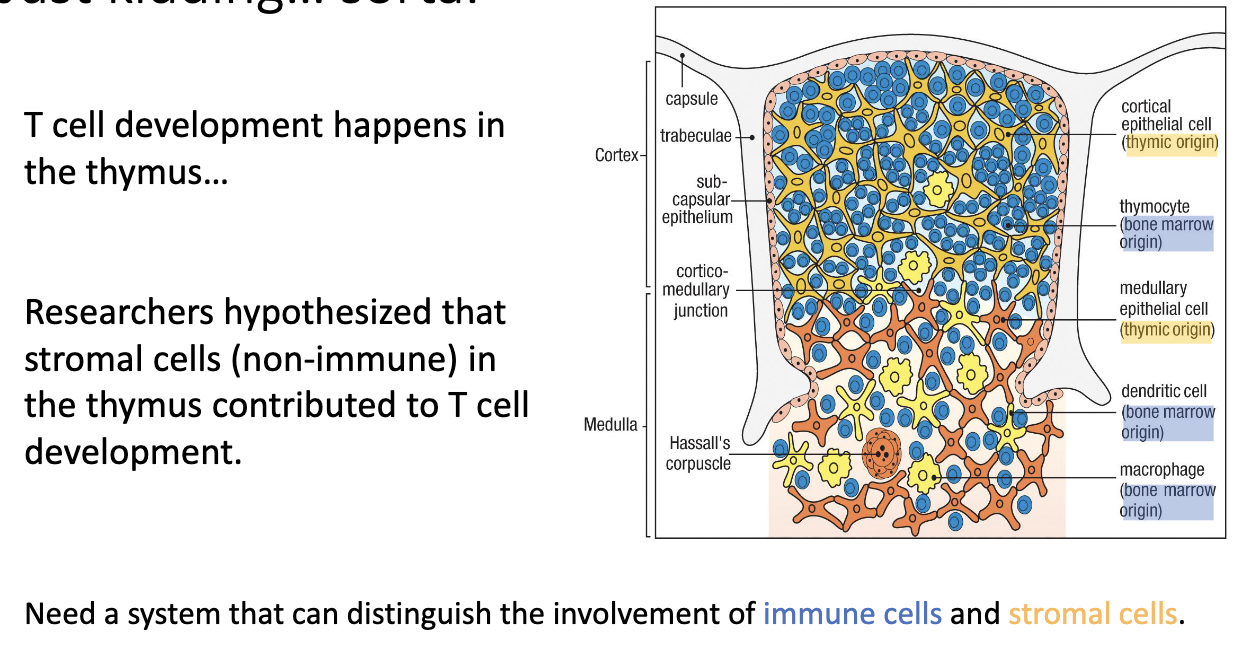
What are the main thymic regions?
Capsule (outer covering)
Cortex (outer dense region – early development)
Blue circles: Developing T cells = thymocytes.
Medulla (inner region – later stages)
How does DiGeorge syndrome show the importance of the thymus for T-cell maturation?
Caused by deletion on chromosome 22.
Missing gene → thymus does not develop.
Without thymus → no mature T cells → severe immunodeficiency.

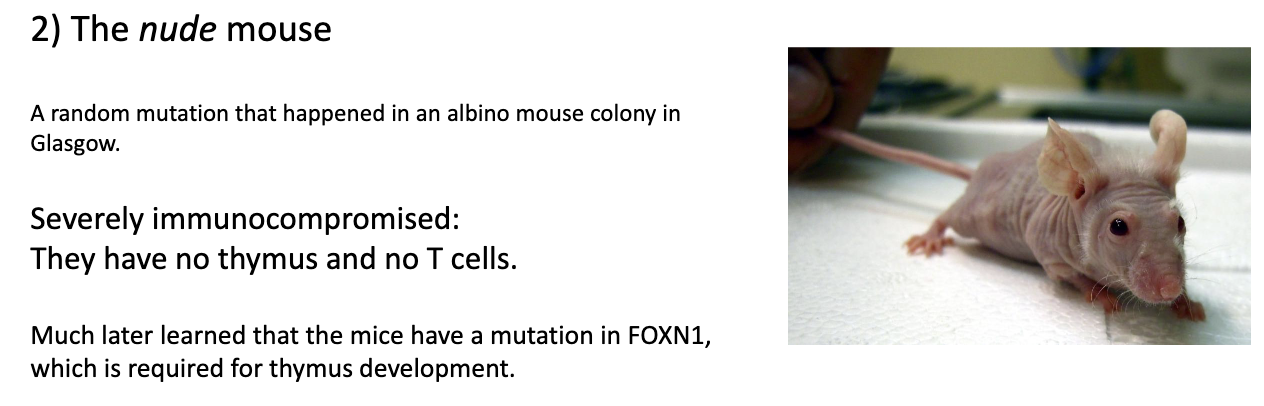
How do nude mice demonstrate the role of the thymus in T-cell maturation?
Nude mouse: spontaneous mutant discovered in albino mice → bred into own strain.
Key feature: no thymus → no mature T cells.
Mutation: in FOXN1 gene, essential for thymus development.
How did Jacques Miller demonstrate the thymus’s role in T-cell development?
Thymectomy in neonatal mice: no T cells develop.
Thymus transplant into nude mice: T cells are restored.

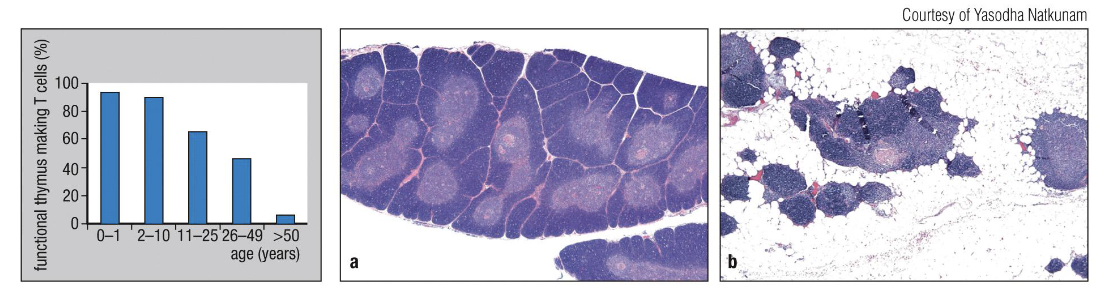
How does the thymus change with age?
It shrinks and fills with fat (thymic involution), so fewer new T cells are made.
Removing the thymus in adults doesn’t affect existing T cells.
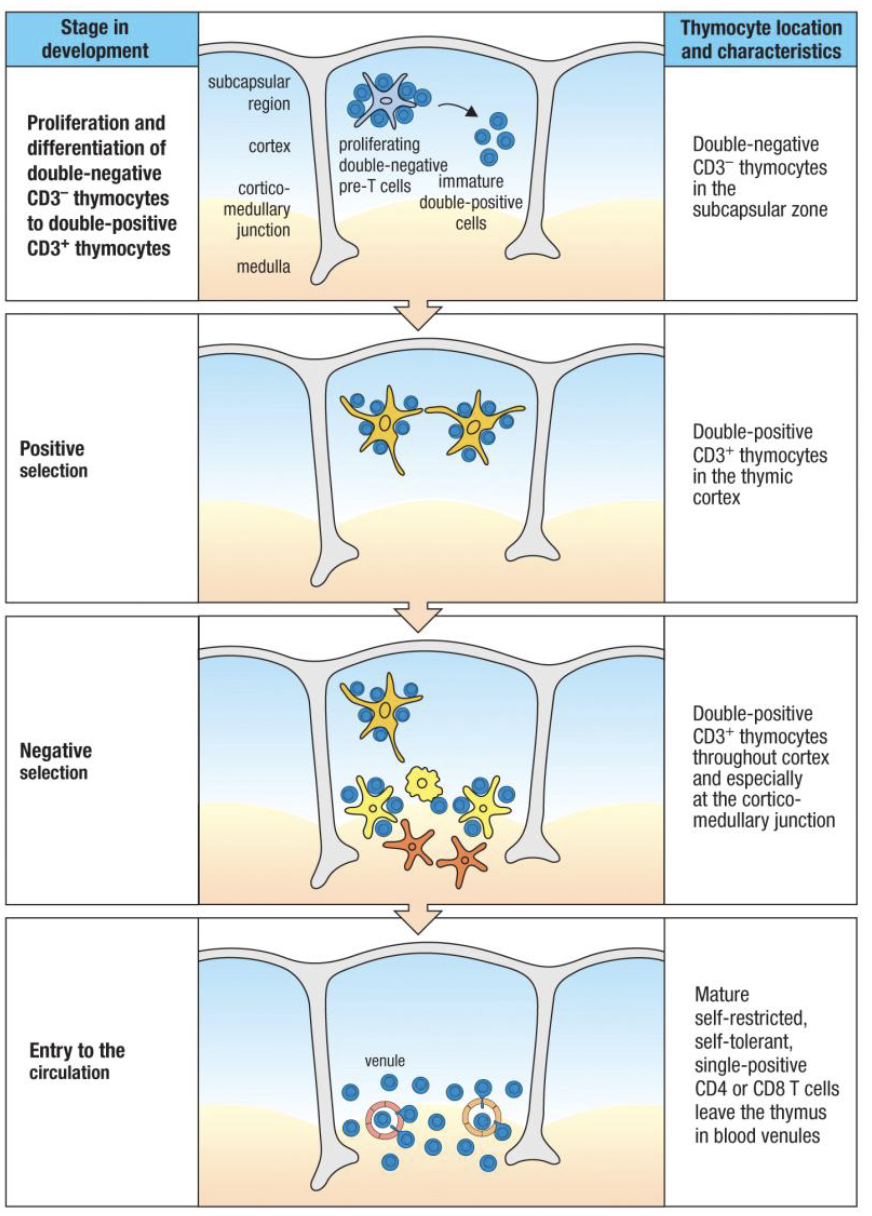
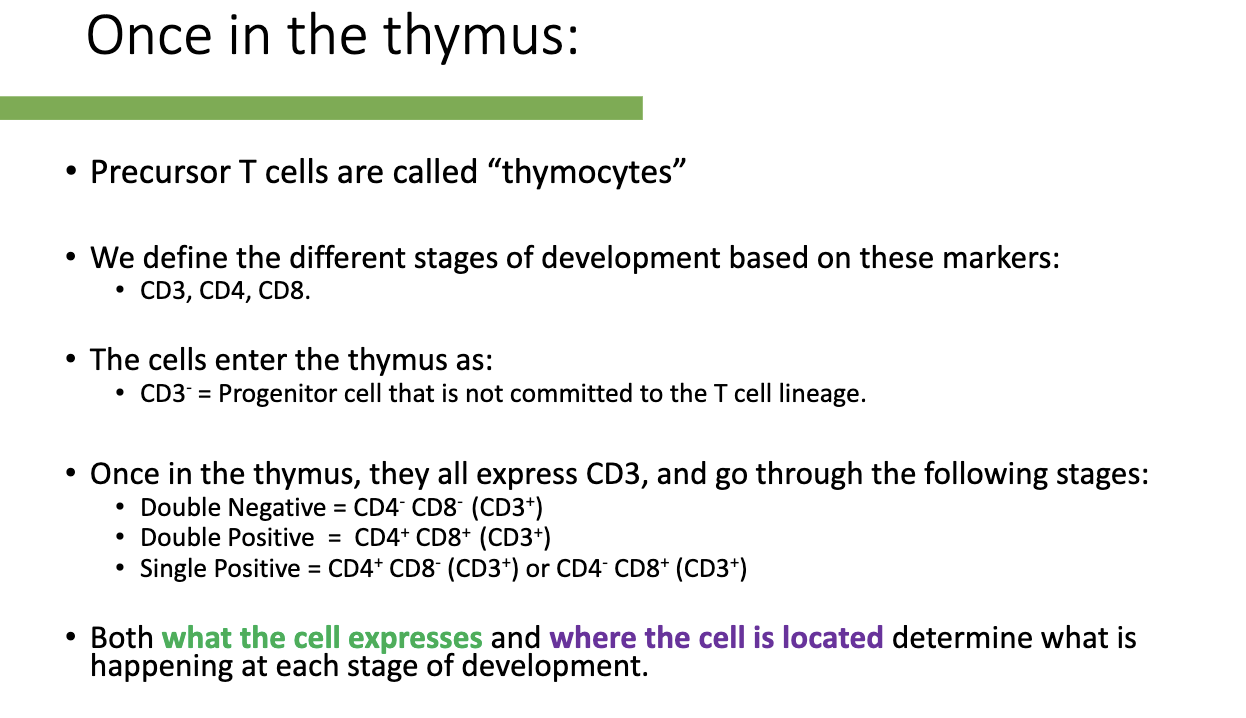
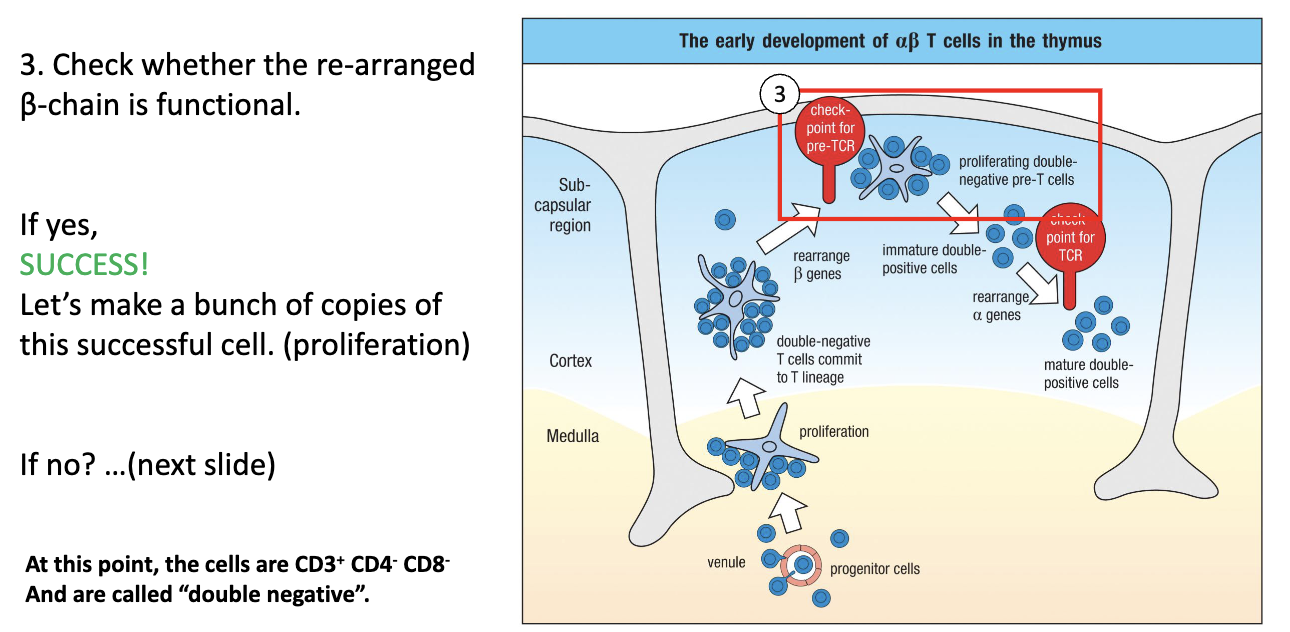
What are the stages of T-cell development in the thymus and how are they defined?
Precursor T cells in thymus: called thymocytes.
Markers used to define stages: CD3, CD4, CD8.
Entry stage: CD3- (uncommitted progenitor).
Development stages (all CD3+):
Double Negative (DN): CD4- CD8-
Double Positive (DP): CD4+ CD8+
Single Positive (SP): CD4+ CD8- or CD4- CD8+
Function depends on: what markers the cell expresses and its location in the thymus.
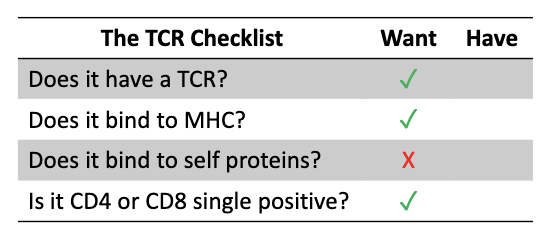
How do thymocyte stages match the TCR “checklist”?
Development follows:
Make TCR
Test MHC binding
Test self-reactivity
Become CD4 or CD8 single positive
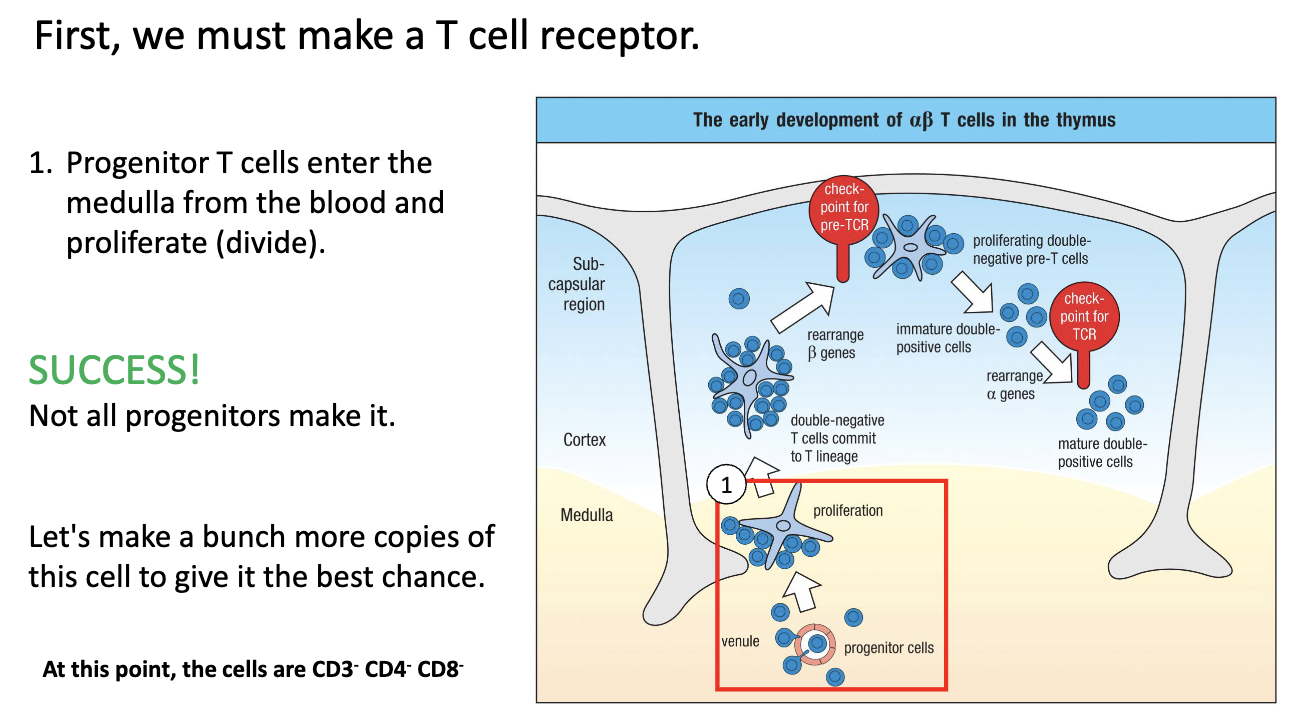
What happens when progenitor T cells first enter the thymus?
Enter thymus via venules into medulla/cortex.
Proliferate to make multiple copies (“save states”) of each progenitor.
Not all cells succeed; only some clones will continue T-cell development.
This expansion allows multiple chances for TCR rearrangement and successful T-cell maturation.
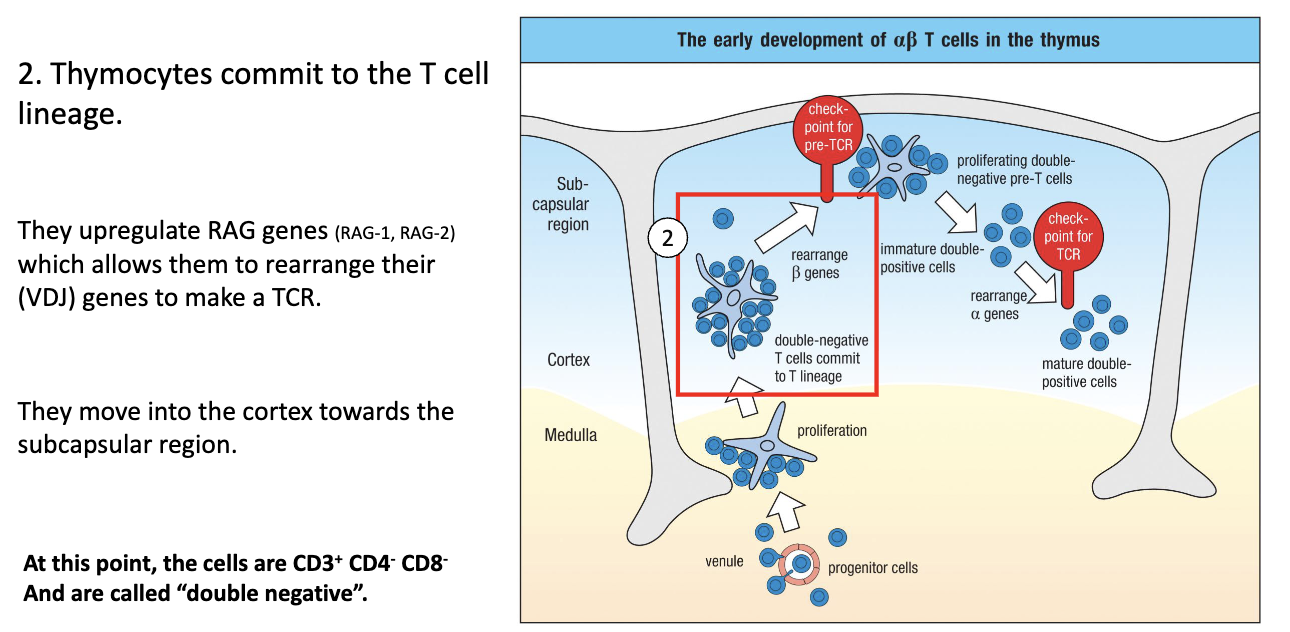
What happens to T cells in the thymic cortex during early development?
Move up into cortex → commit to T-cell lineage.
Turn on CD3 and begin TCR gene rearrangement.
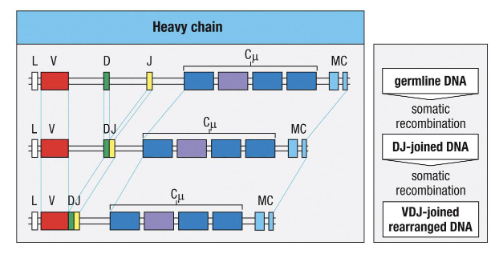
RAG (RAG-1, RAG-2) genes are expressed → mediate VDJ recombination.
DJ joins first, then V joins DJ; specific timing for beta, gamma, delta, alpha chains.
At this stage: Double Negative (CD4- CD8-), CD3+, starting TCR rearrangement.
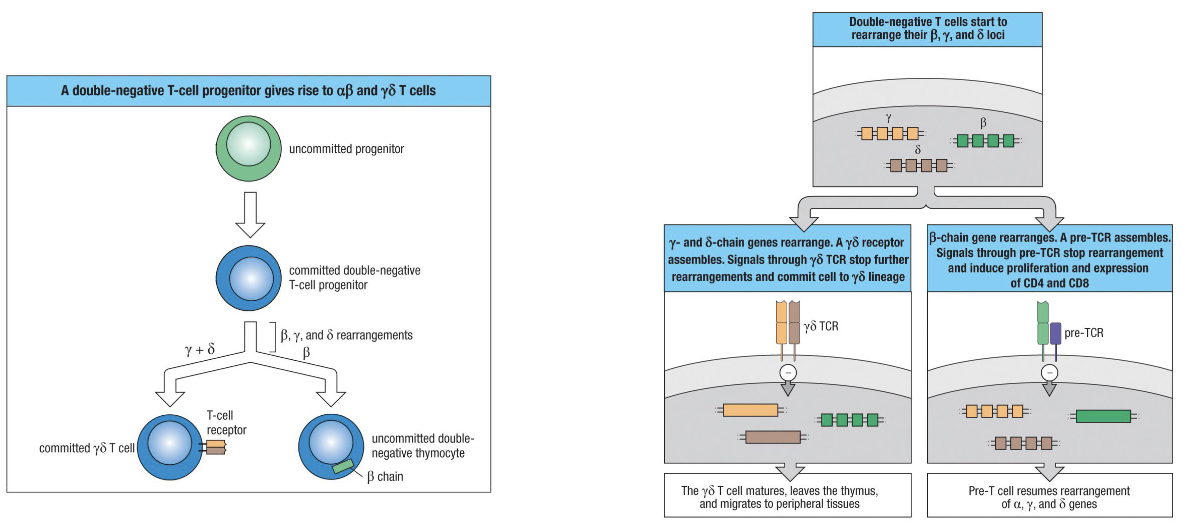
When and how is the αβ vs γδ decision made?
Committed progenitor expresses RAG genes → starts VDJ recombination at gamma, delta, and beta loci (NOT alpha).
Whichever locus rearranges successfully first determines cell type:
γδ TCR first → cell becomes γδ T cell, stops rearranging, leaves thymus mature.
Beta chain first (most common) → pairs with surrogate alpha chain, signals successful TCR → becomes αβ T cell.
TCR signaling required for survival: cells failing to make a functional receptor undergo apoptosis.
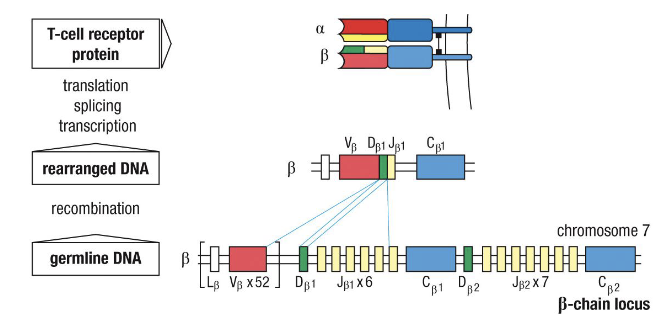
What does “rearranging” TCR genes mean?
VDJ recombination—random joining of gene segments to create unique receptors (same as BCRs).
What are the key facts about αβ vs γδ T cells?
95% of T cells: αβ TCR → majority, main focus in immunology.
γδ T cells:
Rare, mostly at mucosal sites, like intestines
Do not express CD4/CD8
Low diversity (low number of V genes)
Bind non-MHC targets (e.g., stress proteins)
Function more like innate immune cells
What is the next step after T cell lineage commitment?
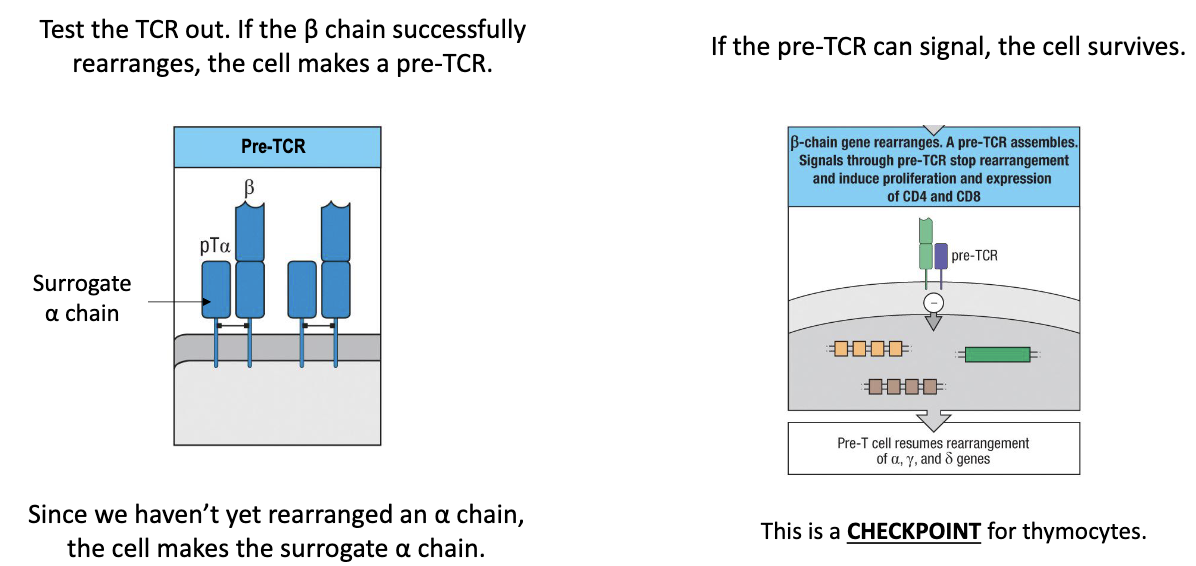
Test whether the rearranged β chain forms a functional pre-TCR.
Still CD3+ CD4– CD8–, i.e. double negative.
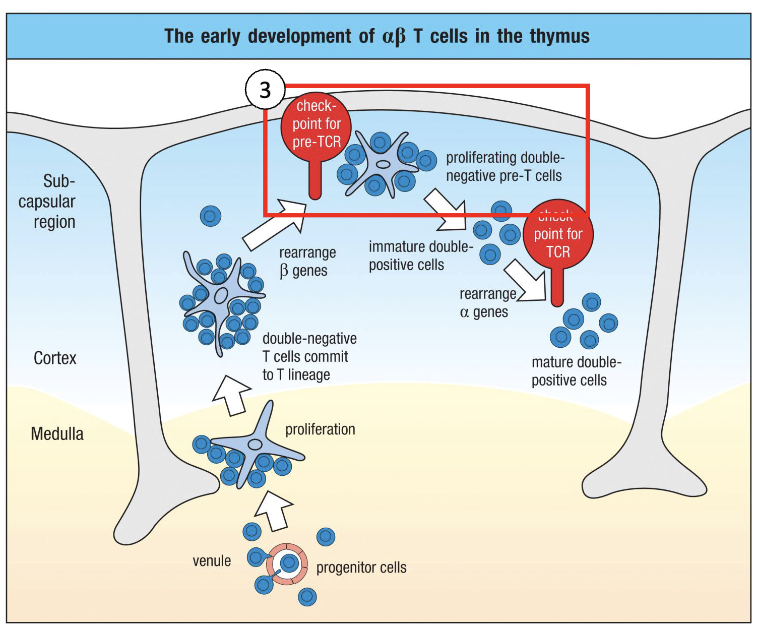
How is the β chain tested to ensure there is a functional TCR?
Surrogate alpha chain pairs with newly rearranged beta chain (before real alpha chain exists).
If beta + surrogate alpha forms a functional receptor → signals the cell → cell survives.
This is a checkpoint: ensures only cells with functional TCR survive.
Successful cells proliferate to make multiple copies (“safe states”) for further development.
What happens if β chain rearrangement works?
The cell proliferates, expanding successful clones (still double negative).
What happens if β chain rearrangement fails?
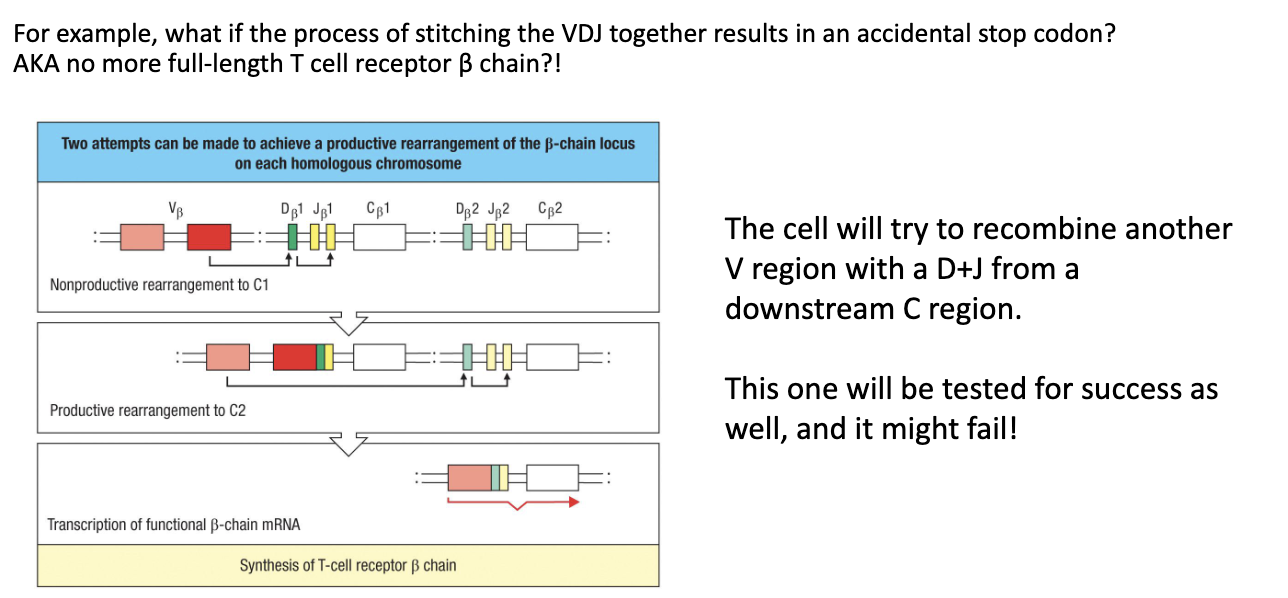
Non-productive rearrangement: cell did not make a functional beta chain.
Retry mechanism: cell tries other V + D + J combinations with alternative beta chain constant regions.
2 attempts: cell has several chances to make a functional beta chain (2 alleles: 1 from each parent but each allele can try multiple VDJ combinations).
Outcome: if successful → cell survives and proliferates; if not → cell eventually dies.
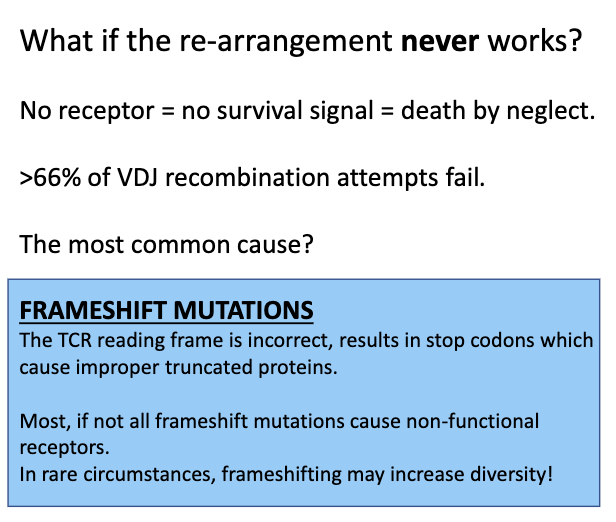
What if the β chain re-arrangement never works?
Randomness in VDJ recombination → may produce frame shifts or non-productive sequences.
No functional TCR → no survival signal → cell dies (“death by neglect”).
Failure rate: ~66% of VDJ rearrangements fail.
Importance of proliferation: multiple “safe state” copies increase chances that at least one cell succeeds.
What happens when T cells rearrange their alpha chain, and why do they become double positive?
Alpha chain rearrangement: V + J segments (no D).
Surrogate alpha chain is replaced by functional alpha chain on the surface.
Functional alpha-beta TCR signals → cell survives.
CD4 and CD8 are turned on → cell becomes Double Positive (DP).
Reason for DP expression: needed to test the alpha-beta TCR with MHC I and II during positive selection.
Location: cells move up to cortex, then start moving back down toward medulla.
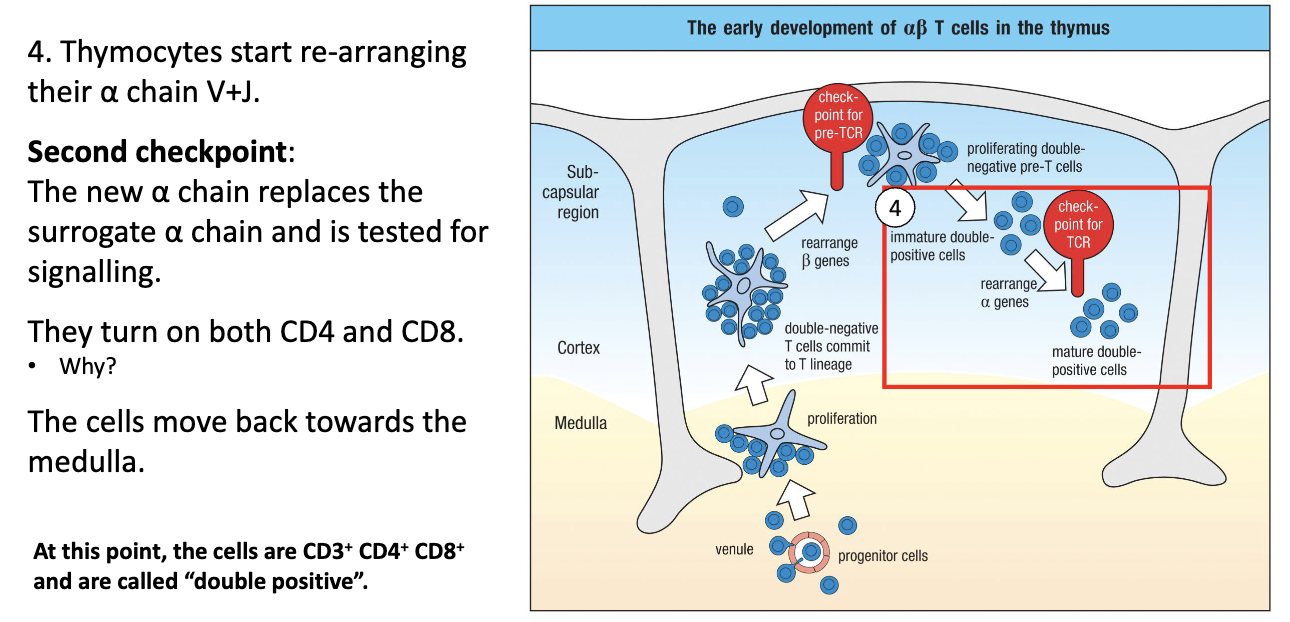
What is true of double positive (DP) thymocytes?
Have functional TCR
Express both CD4 and CD8
Next tests: MHC binding and self-reactivity
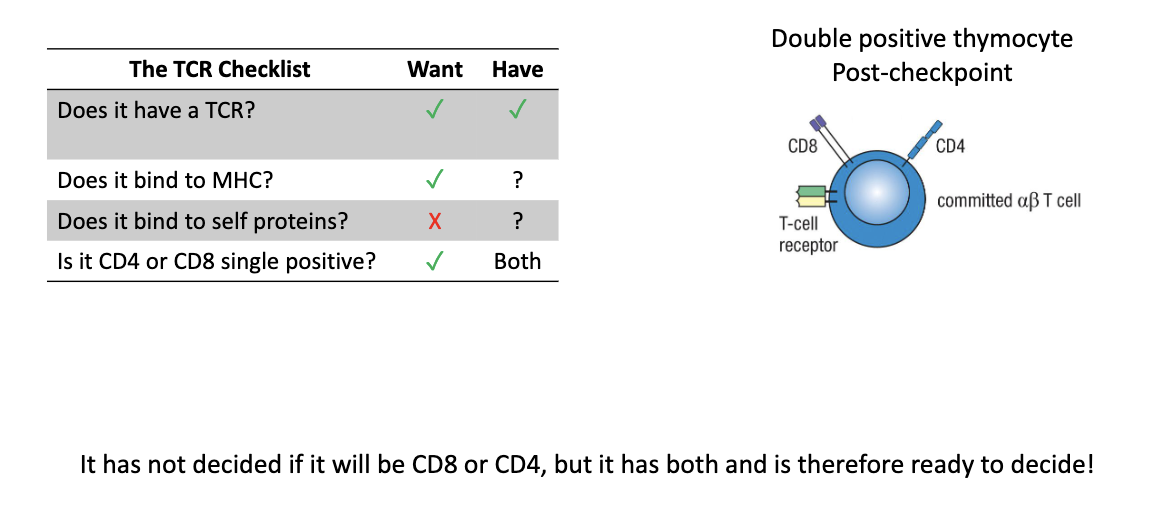
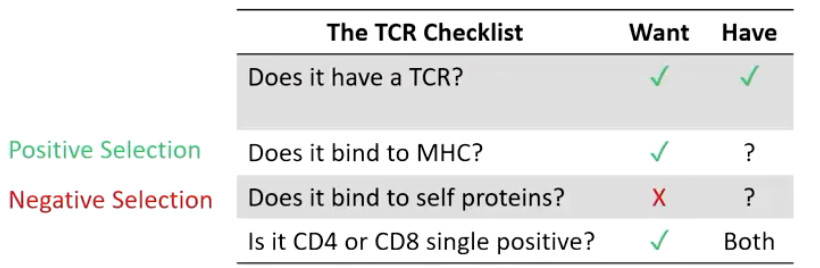
What two processes refine the TCR repertoire?
Positive selection – keep T cells that bind MHC
Negative selection – remove self-reactive T cells
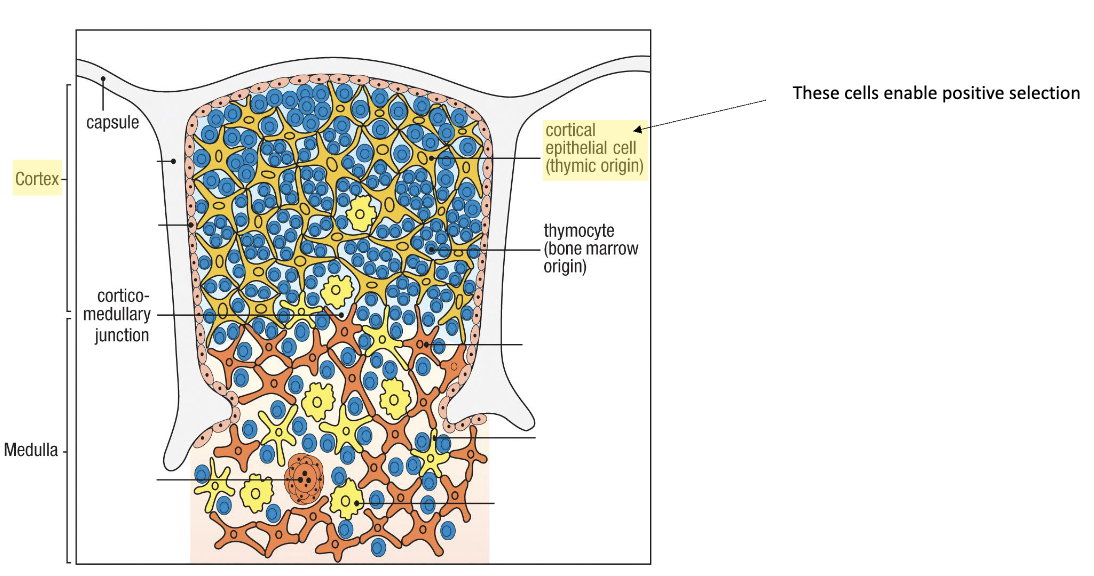
Where does positive selection occur?
In the cortex of the thymus, mediated by cortical epithelial cells (not immune cells).
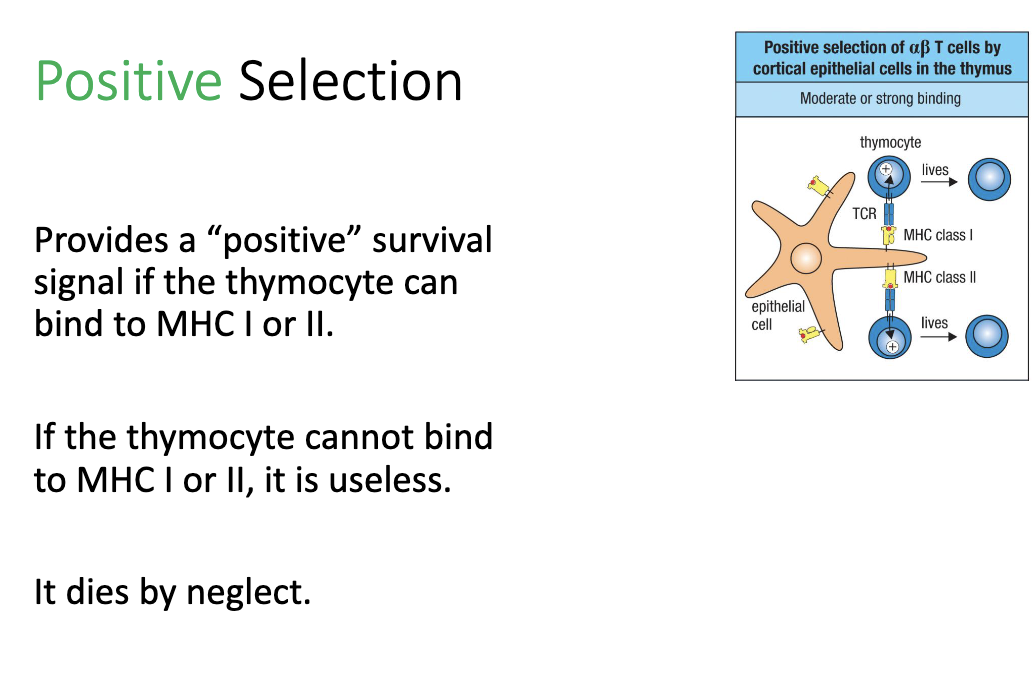
What happens during positive selection in the thymus?
Thymocyte tests TCR binding to MHC I or II.
Moderate to strong binding: receives positive survival signal → cell lives.
No binding: fails positive selection → dies by neglect.
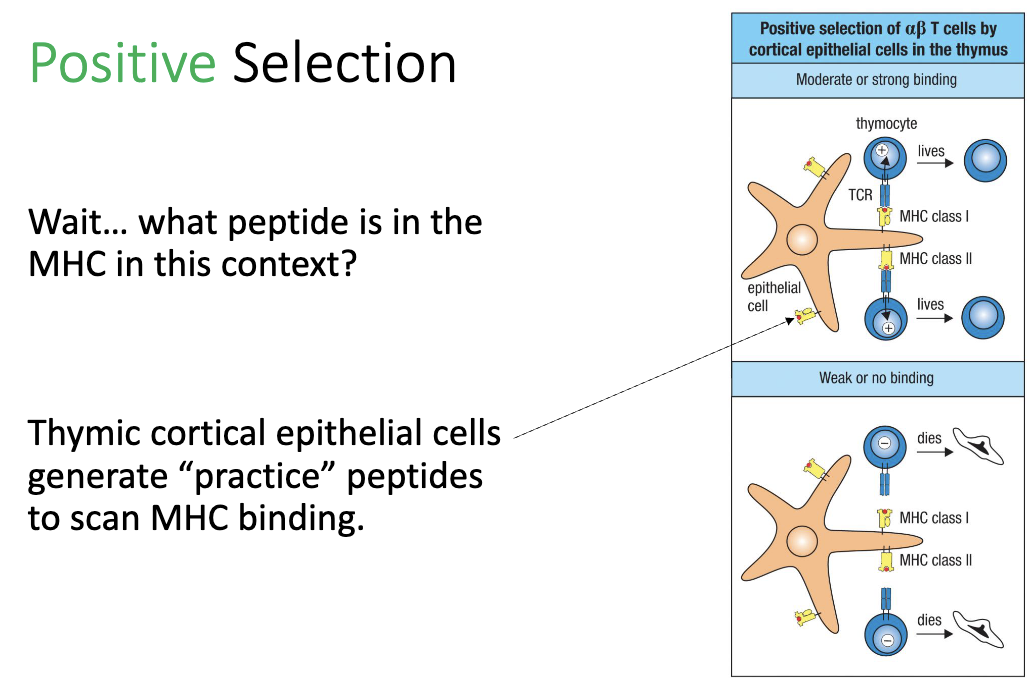
What peptides are presented during positive selection?
Thymic cortical epithelial cells load “practice peptides” on MHC
Purpose: T cells are testing MHC binding, not specific peptide recognition.
How was it discovered that T-cell development depends on thymic stromal cells?
Researchers (e.g., Jacques Miller) questioned why T-cell development must occur in the thymus.
Observations:
Mice with a thymus (even transplanted elsewhere) → still made T cells.
Mice without a thymus → no T cells.
Hypothesis: Some thymic stromal cells (non-immune cells) are essential for T-cell development.
To test this, they used chimeric mice (mix of different cell types) to distinguish whether immune cells or stromal cells were responsible.
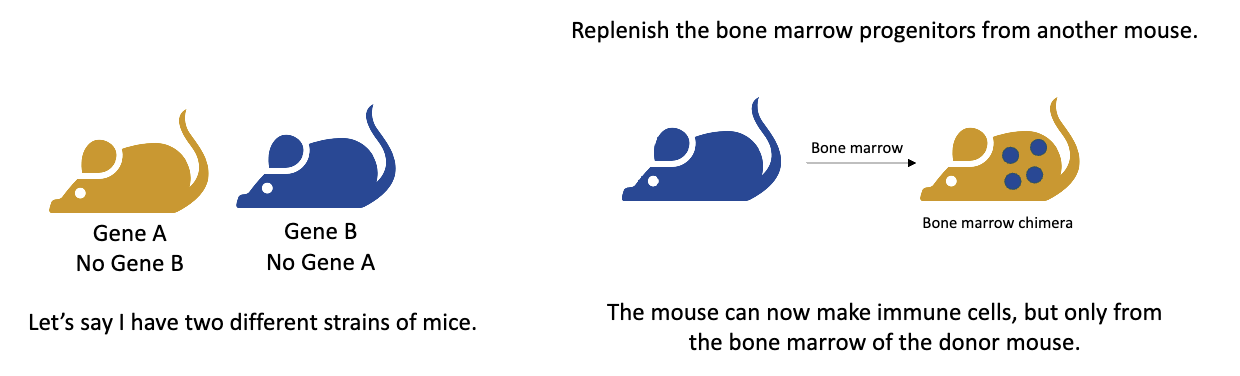
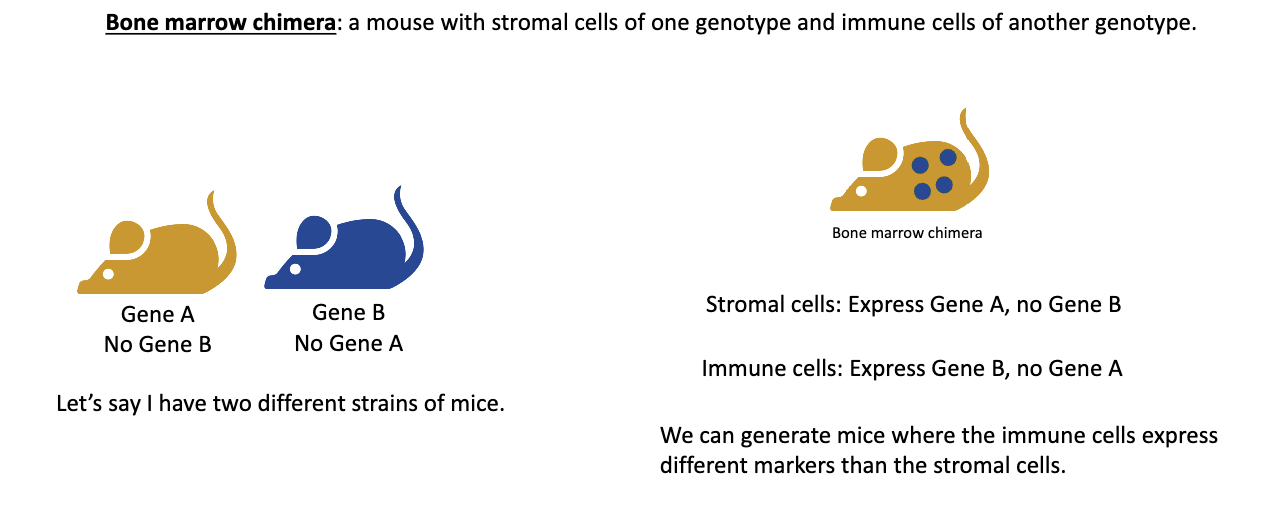
What is a bone marrow chimera and how is it made?
A bone marrow chimera is a mouse whose immune system comes from a different donor mouse’s bone marrow.
Steps:
Two mouse strains are used — one has gene A (no gene B) and the other has gene B (no gene A).
The recipient mouse is irradiated, which destroys its bone marrow progenitor cells, wiping out its immune system.
Bone marrow from the donor mouse is transplanted into the irradiated mouse.
The new immune cells develop from the donor bone marrow, while the stromal (structural) cells remain from the original host.
Example of bone marrow transplantation to create a bone marrow chimera.
Example: If a gene A mouse receives bone marrow from a gene B mouse →
Stromal cells (non-immune) = gene A
Immune cells = gene B
This setup lets scientists test whether immune functions depend on immune cells or stromal cells, making it a powerful tool in immunology.
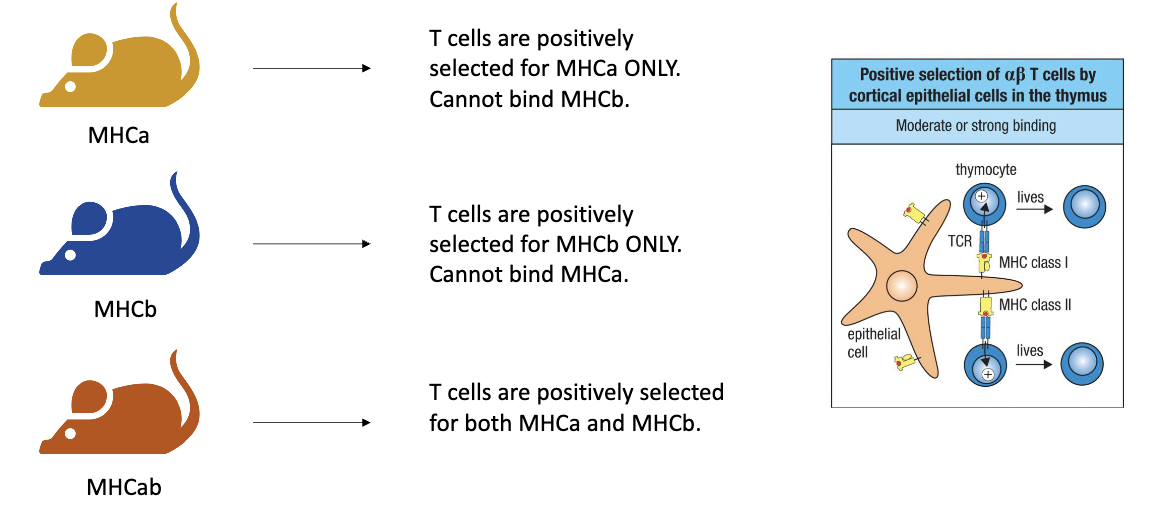
How did researchers use MHC variants in bone marrow chimera experiments to study T-cell development?
There are different types of MHC class II molecules (e.g., MHCA and MHCB).
Researchers used two mouse strains:
One mouse had MHCA only.
The other had MHCB only.
By crossing the two strains, they produced:
Homozygous mice → only MHCA or only MHCB.
Heterozygous mice → both MHCA and MHCB.
These mice were then used in bone marrow chimera setups to test whether T-cell selection depends on MHC type expressed by stromal (thymic) cells or by bone marrow–derived immune cells.

How does positive selection differ in MHCA, MHCB, and heterozygous (MHCA+MHCB) mice?
MHCA-only mice: T cells are positively selected for MHCA only → their TCRs can bind MHCA but not MHCB.
MHCB-only mice: T cells are positively selected for MHCB only → their TCRs can bind MHCB but not MHCA.
Heterozygous (MHCA+MHCB) mice: T cells can be selected for either MHCA or MHCB, producing a mix of TCR specificities for both MHC types.
But they didn’t know that at the time…
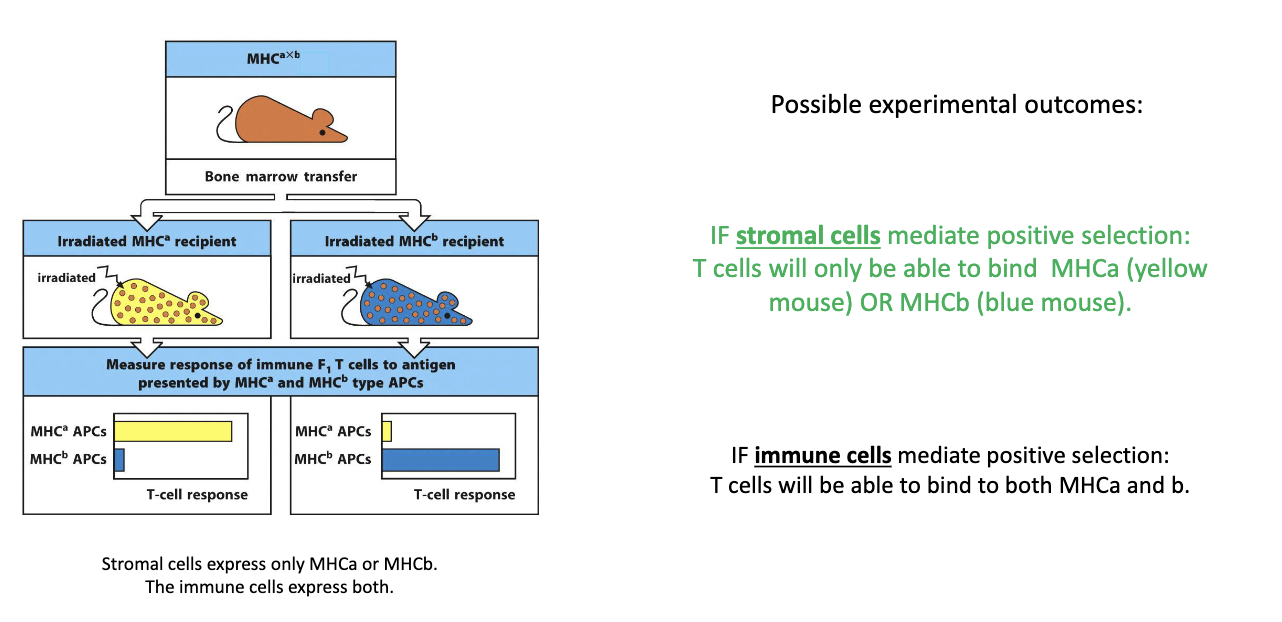
What did the bone marrow chimera experiment reveal about which cells mediate positive selection of T cells?
Setup:
Stromal cells express either MHCA or MHCB
Immune cells express both MHCA and MHCB
Predictions:
If stromal cells drive positive selection → T cells should only recognize either MHCA or MHCB type of the stromal cells.
If immune cells drive it → T cells should recognize both MHC types.
Result:
In MHCA-only mice → T cells recognized only MHCA.
In MHCB-only mice → T cells recognized only MHCB.
Conclusion: Positive selection is mediated by thymic stromal (epithelial) cells, not immune cells.
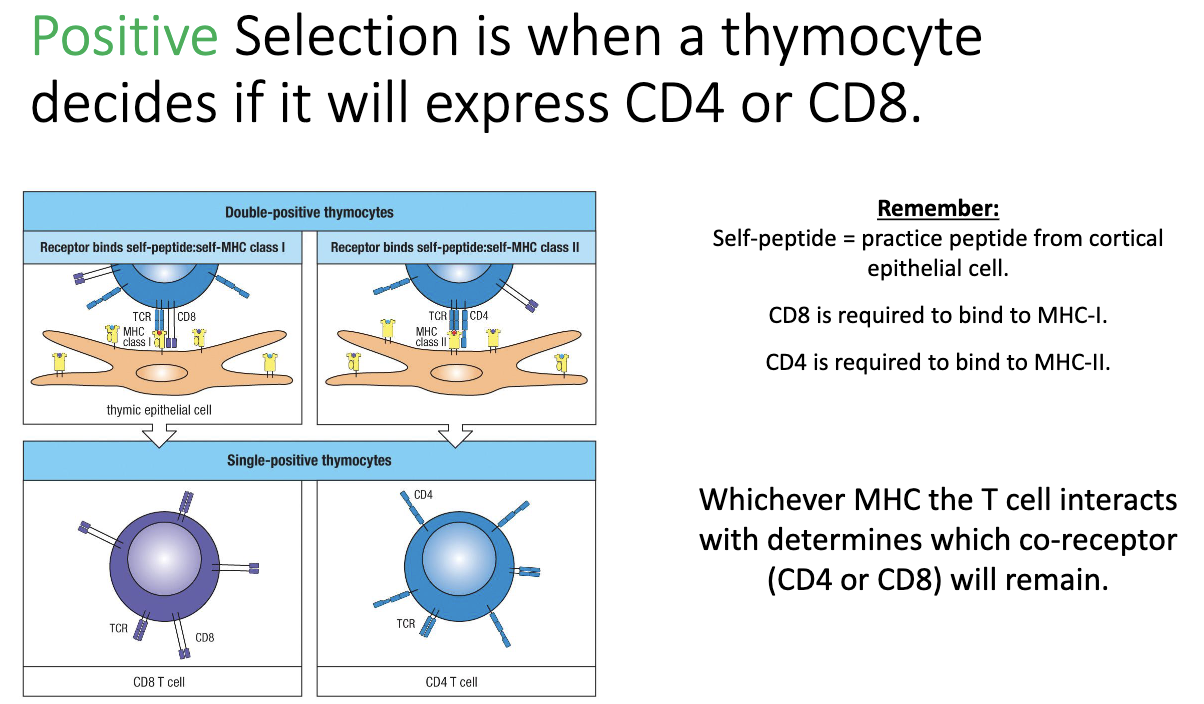
How does positive selection determine CD4 vs CD8 expression in thymocytes?
During positive selection, thymocytes test their TCR binding to MHC molecules:
Binds MHC I → keeps CD8
Binds MHC II → keeps CD4
Cannot bind either → dies
Outcome: single positive (SP) thymocyte expressing either CD4 or CD8.
At this stage: TCR is functional and binds MHC, but self-reactivity has not yet been tested.
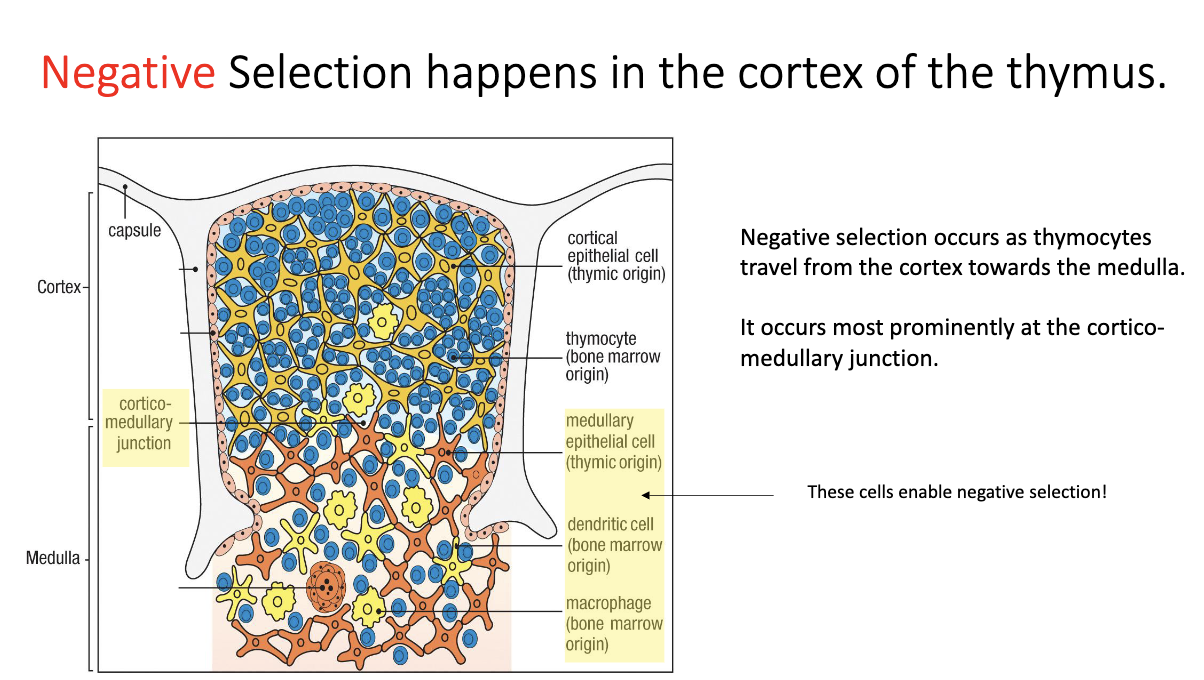
Where does negative selection occur in the thymus and what are the key cells involved?
Location: at the cortex–medulla junction of the thymus.
Key cells involved: medullary epithelial cells, dendritic cells, macrophages.
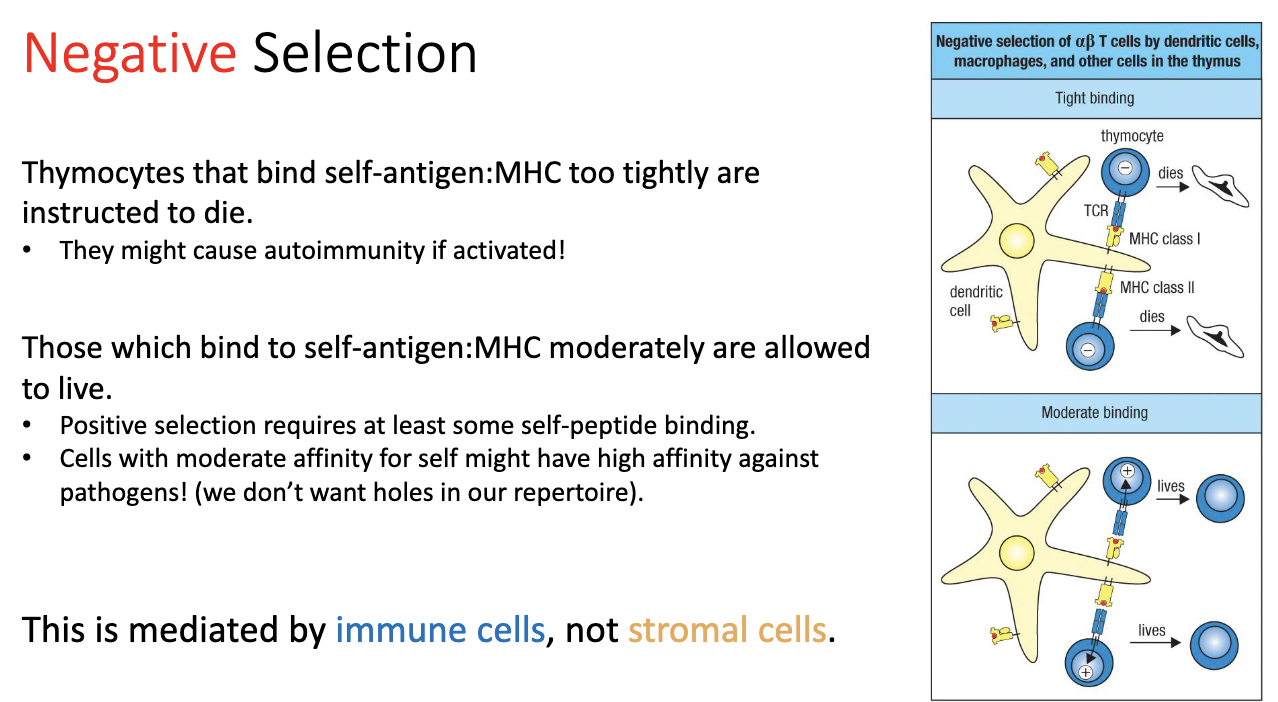
How does negative selection work, and what determines T cell survival or death?
Process:
T cells encounter self-peptides on MHC.
Strong binding → cell receives a death signal → eliminated.
Moderate binding → cell survives → allowed to continue (needed for proper positive selection).
Weak/no binding → cell would not have survived positive selection anyway.
Reason for threshold:
Ensures T cells recognize self-MHC but do not strongly react to self-peptides.
Maintains self-tolerance while avoiding gaps that pathogens could exploit by mimicking self.
Mediators: immune cells, not stromal cells.
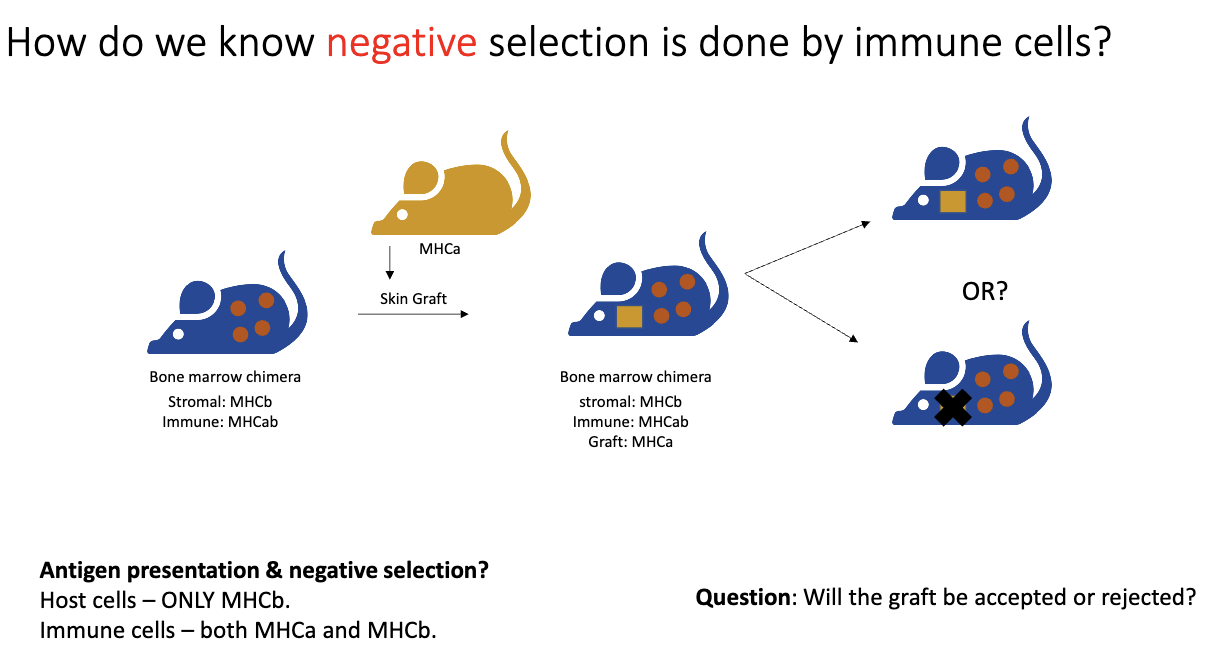
How do we know that negative selection is mediated by immune cells, and what role does MHC play in skin graft experiments?
Negative selection: removes T cells that strongly react to self-peptides.
Evidence from skin grafts:
Genetically identical mice (same MHC, e.g., MHCA → MHCA): skin grafts are accepted because self-reactive T cells were removed by negative selection.
Genetically different mice (different MHC, e.g., MHCA → MHCB): skin grafts are rejected because T cells recognize the foreign MHC.

How did the bone marrow chimera experiment test whether negative selection is mediated by immune cells?
Setup:
Recipient mouse stromal cells: MHCB only.
Donor bone marrow (immune cells): MHCAB.
Test:
Skin graft from MHCA mouse (yellow) placed on recipient (blue, MHCB stromal cells).
Logic:
Normally, MHCA graft → rejected by T cells.
If negative selection is mediated by immune cells, T cells should be tolerized to MHCA because the immune cells express it.
Outcome shows whether immune cells can induce tolerance versus stromal cells.
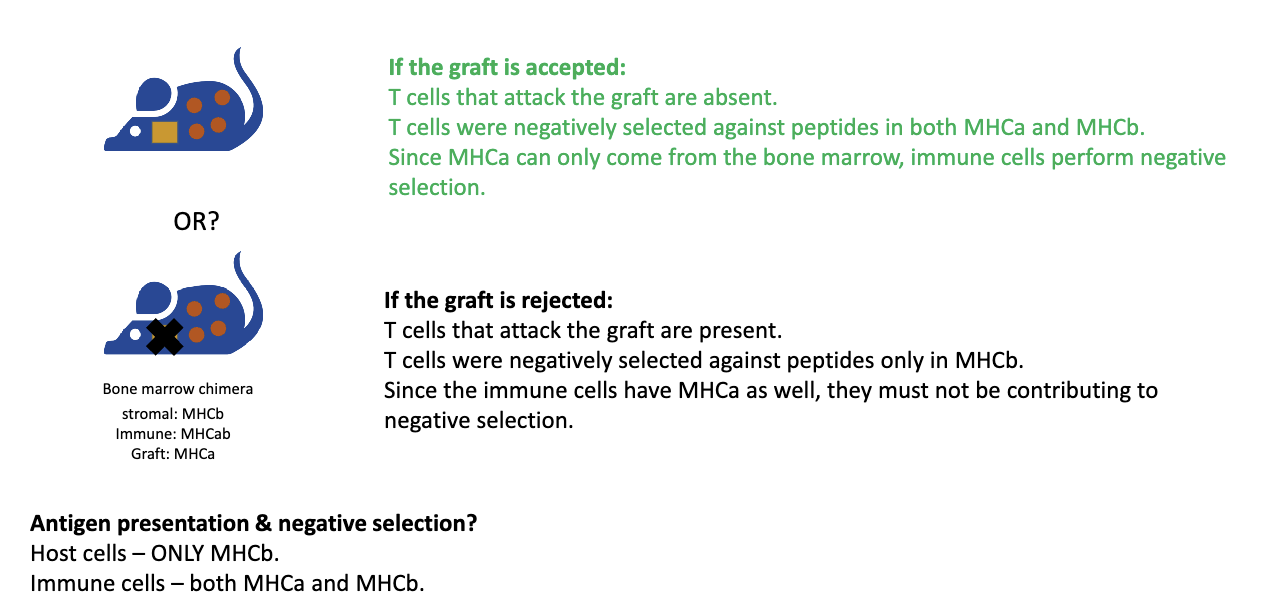
What were the results of bone marrow negative selection experiment?
Skin graft from MHCA mouse tested:
Outcome: Graft accepted → T cells recognizing MHCA removed → negative selection done by immune cells (not stromal cells).
If graft rejected → immune cells not mediating negative selection.
Result: Graft was accepted!
Key points:
Positive selection is mediated by stromal/epithelial cells.
Negative selection primarily by dendritic cells/macrophages (immune cells).
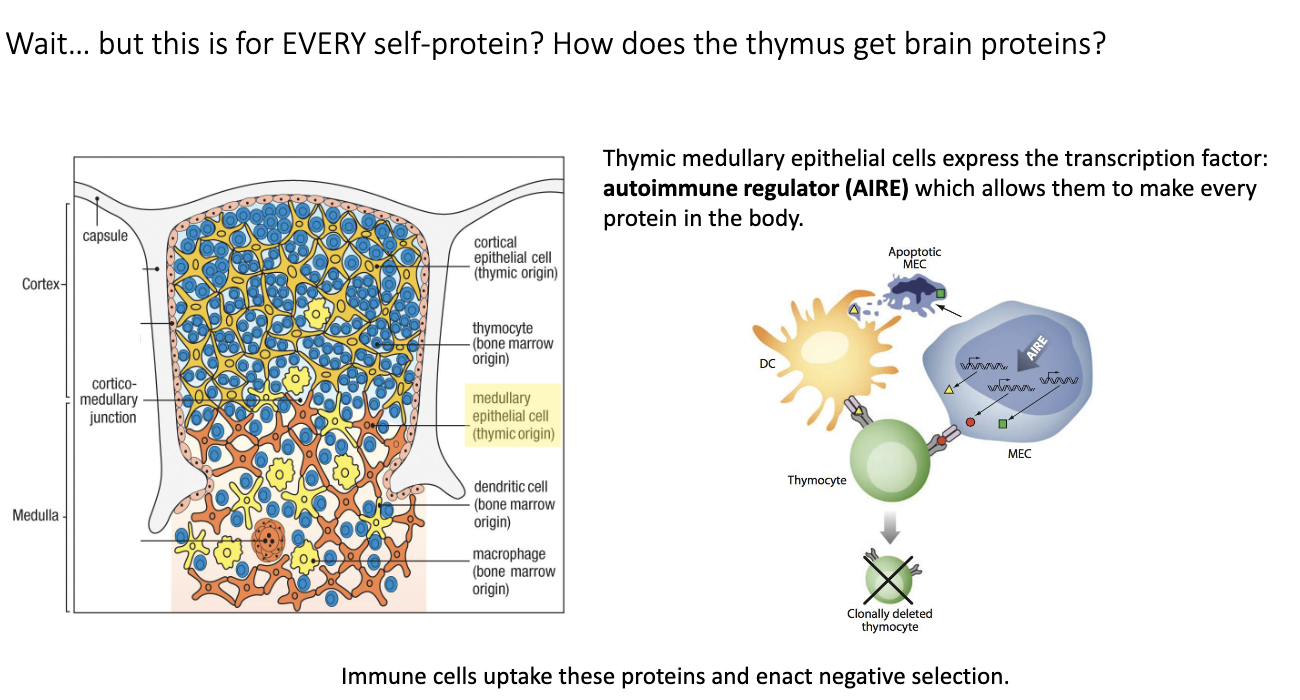
How do medullary thymic epithelial cells and the AIRE transcription factor contribute to negative selection of T cells?
Problem: Thymocytes need to be negatively selected against tissue-specific proteins (e.g., brain peptides), but thymus is not that tissue.
AIRE (Autoimmune Regulator):
Transcription factor in medullary thymic epithelial cells (mTECs).
Drives expression of many tissue-specific genes in thymus.
AIRE knockout → autoimmunity.
Mechanism:
mTECs produce self-proteins → dendritic cells uptake them → present on MHC → T cells that bind too strongly are deleted.
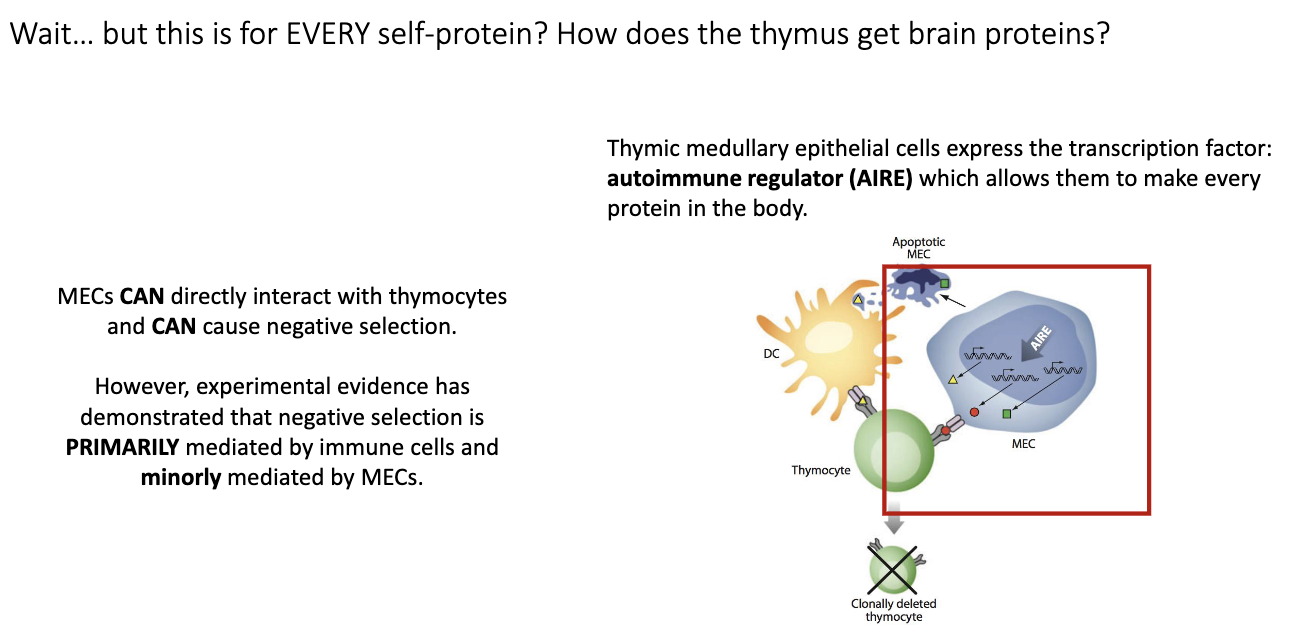
mTECs can also directly present to thymocytes, but mainly dendritic cells mediate negative selection.
Outcome:
Clonal deletion of self-reactive T cells.
Prevents autoimmunity while allowing functional T cells to survive.
Who performs most negative selection?
Mainly immune cells (like dendritic cells), though AIRE+ medullary epithelial cells can also contribute.
What proportion of thymocytes survive thymic selection?
98% of thymocytes die.
Thymocyte numbers:
~100 million total at any time.
~50,000 new thymocytes enter per day.
Only ~1 million successfully exit the thymus.
Reasons for death:
Failure to make productive VDJ rearrangements.
TCR cannot bind MHC.
TCR binds too strongly to self-peptides → negative selection.

Summarize T cell development in order.
Bone marrow: T cell precursors form
Thymus entry: proliferation (DN)
β & α chain rearrangement → TCR
Positive selection (cortex): keep MHC-binders
Lineage choice: CD4 or CD8
Negative selection (medulla): remove self-reactive
Naïve single-positive T cells exit to lymphoid tissues