Chem 13 and 14 vocab for final
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
Heat
The transfer of energy from a warmer place to a colder place
Kilocalories (Kcal)
1000 calories, also known as Nutritional or big “C” Calorie
Joule
SI unit for energy/heat a 1cal=4.184 J. Must use this unit for heat equation
“m”
(grams). Symbol that represents the amount of “MASS” in the heat equation
Specific heat
(j/g^degree C). Amount of energy required to raise 1 g of ANY substance in 1 degree C
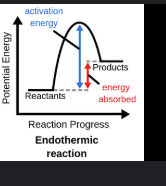
“ENDO”thermic
Rezction where energy (heat) is required. The environment feels colder (ICE PACK)
Calorimeter
Insulated device, water measures heat gain’oss when substance burns
positive enthalpy (H+)
Heat is gained during a chem reaction (ENDO). Products higher than reactants
energy
ability to do work or produce heat
“c” (from heat equation)
(j/g^degree c). Symbol that represents the “specific heat” in the heat equation
Calculating the heat equation
“q”=”m”x”c”x”T”
q=mcat
Q=j
m=g
c=j/g^degree c)
Kj (Kilojoule)
1000 joules
“c”alorie (small “c”)
amount of energy needed to raise ine g of WATER 1 degree celsius
(ΔT) (from heat equation)
symbol that represents “change in Temp” in the heat equation
Chemical potential energy
Energy that is locked in the bonds of chemical substances (food, gas, etc).
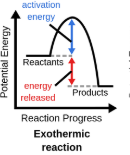
“EXO”thermic
Reaction where energy (heat) is given off. Environment feels warmer (Hand warmers)
water
substance with a very high specific heat. Absorbs heat without quick rise in Temp.
negative enthalpy (H-)
Heat is LOST during a chemical reaction (EXO). Reactants higher than products
“q”
(J) symbol for the amount of heat gained or lost in the heat equation
Law of Conervation of Energy
States that energy can neither be created nor destroyed but it can be transformed EX: mechanical in electrical , solar into electrical
Endothermic
Postive Enthaply, H+, heat gained, products higher than reactants, environment feels colder, cold pack
Exothermic
Negative enthalpy, H-, heat lost, reactants higher than products, enviroment feels warm, hand warmer
Endothermic graph
Exothermic graph
Catalyst
A substance that increases the rate of a reaction, but does not get "used" during the reaction is known as a
Acitvated Complex
A temporary unstable arrangement of atoms in which old bonds are breaking and new bonds are forming is called a/an
A catalyst _____ the activation energy required for a reaction to take place
decreases
An inhibitor ____ the activation energy required for a reaction to take place
Increases
What increases the rate of reaction
decreasing the activation energy, adding a catalyst, increaing the concentration of the reactants, increasing the temperature
The rate of reaction depends on
colliosn energy, collison frequency, and collison orientation
As the temperature of a reaction is increased, the rate of the reaction increases because the __________.
reactant molecules collide more frequently and with greater energy per collision
A catalyst can increase the rate of a reaction __________.
by providing an alternative pathway with a lower activation energy
A burning splint will burn more vigorously in pure oxygen than in air because
oxygen is a reactant in combustion and concentration of oxygen is higher in pure oxygen than is in air.