Unit 1 Organic Chemistry, Macromolecules, and Chemical Reactions Overview
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
What is Organic Chemistry?
The study of compounds that contain bonds with carbon atoms.
Why is carbon considered the element of life?
Because all living things contain carbon and it can form strong covalent bonds with other elements.
What are the four elements that carbon commonly bonds with to form biological molecules?
Hydrogen, Oxygen, Nitrogen, Phosphorus, and Sulfur.
How many valence electrons does carbon have?
Four valence (outer) electrons.
What types of structures can carbon form?
Chains and ring structures, and it can form single, double, and triple chemical bonds.
What are reactants and products in a chemical reaction?
Reactants are the starting materials that combine to form products.
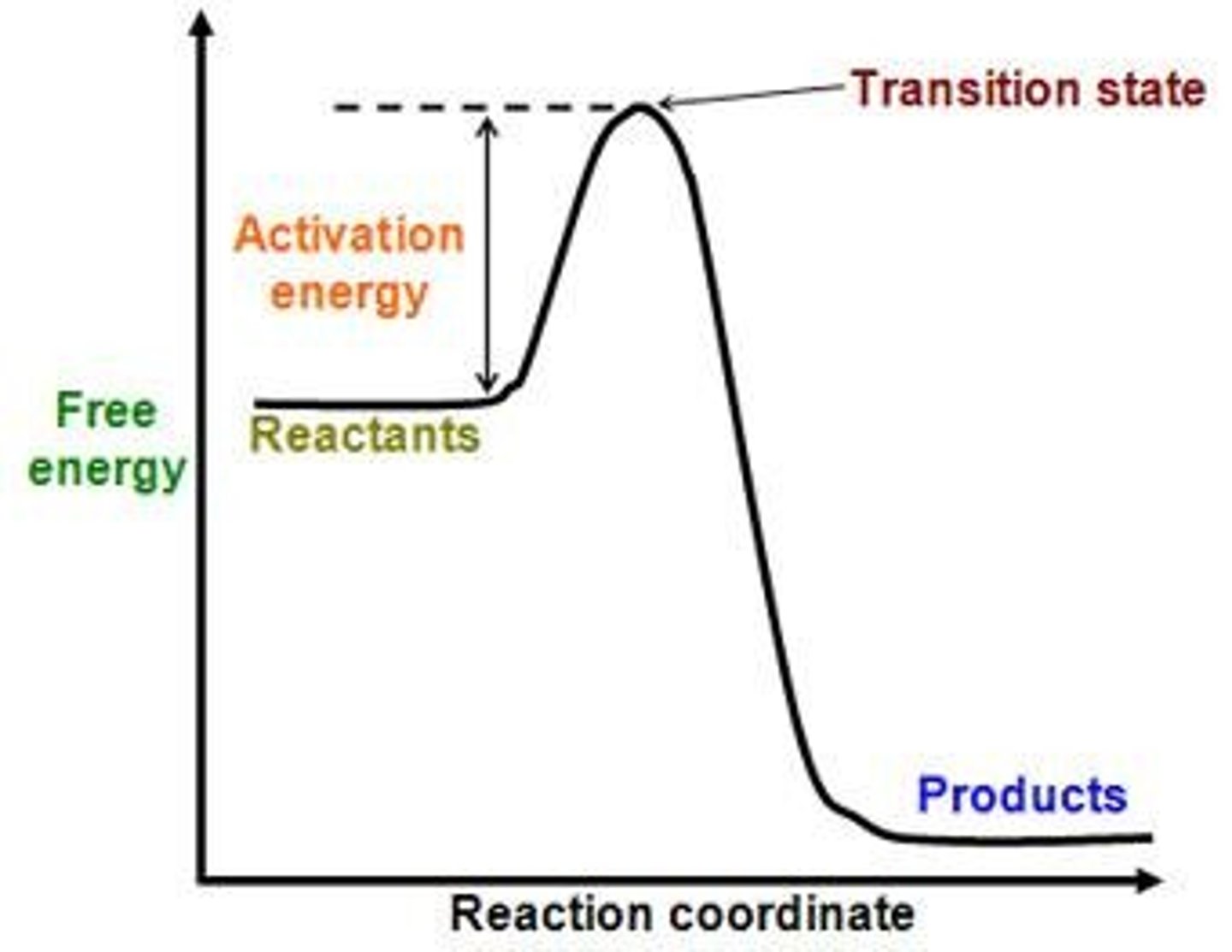
What is activation energy?
The energy needed to start a chemical reaction.
What are macromolecules?
Large organic compounds formed from hundreds or thousands of smaller molecules called monomers.
What is a monomer?
A single molecule of a carbon compound.
What is polymerization?
The process of joining smaller monomers to create larger molecules called polymers.
How is a beaded necklace analogous to a polymer?
Individual beads represent the monomers, while the completed necklace represents the polymer.
What is dehydration synthesis?
The process where monomers are joined together through covalent bonds to make polymers, with water lost as a byproduct.
What is hydrolysis?
The process where polymers are broken down into monomers using water molecules to break the bonds.

What is the difference between anabolic and catabolic reactions?
Anabolic reactions build larger molecules from smaller ones, while catabolic reactions break down larger molecules into smaller ones.
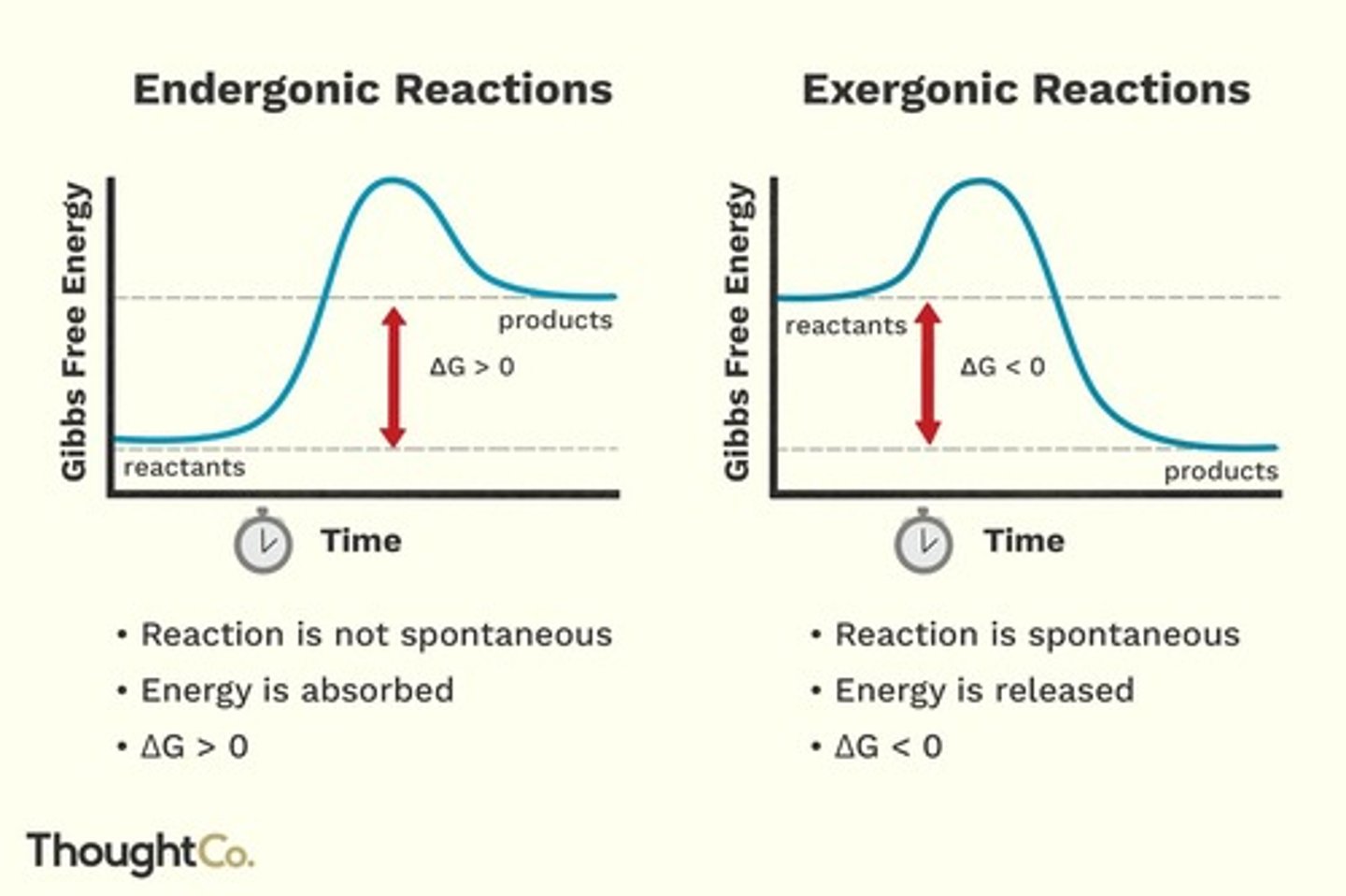
What are endergonic and exergonic reactions?
Endergonic reactions require energy input, while exergonic reactions release energy.