Synaptotagmin Syntaxin Article Worksheet
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
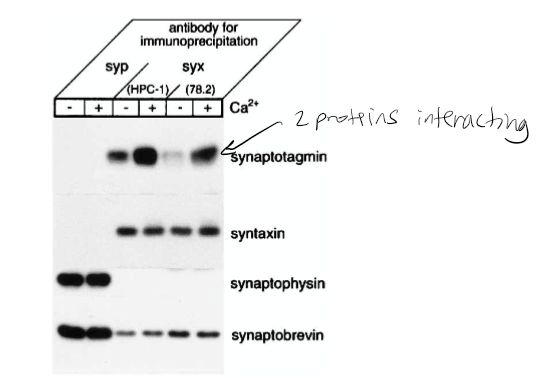
Figure 1: What experiment was conducted?
Pull-down assay immunoprecipitation. Goal if there was a relationship/interaction between syntaxin and synaptobrevin. The experiment conducted was the coprecipitation of synaptotagmin with syntaxin from rat brain detergent extracts that were stimulated by calcium. Syntaxin 1 was immunoprecipitated from the rat brain detergent in the experiment.
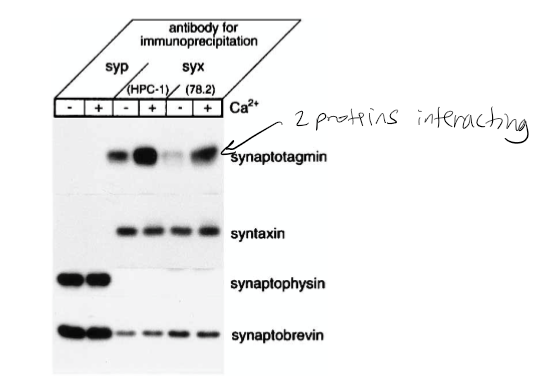
Figure 1: What do the abbreviations “syp” and “syx” stand for?
Syp = synaptophysin, syx = syntaxin 1
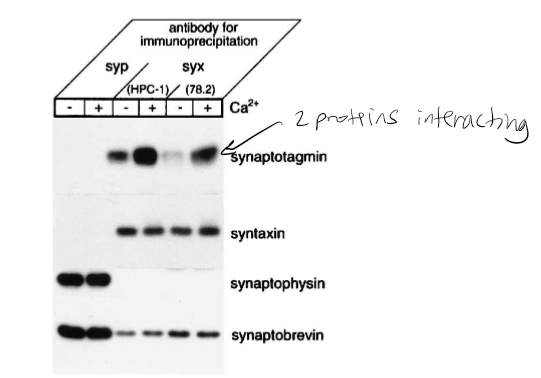
Figure 1: What are “HPC-1” and “78.2”?
Antibodies. HPC-1 is recognized as both syntaxin 1A and 1B. The 78.2 refers to Cl 78.2, which is a new antibody that can be used against recombinant full-length syntaxin 1A and will also recognize syntaxin 1A and 1B.
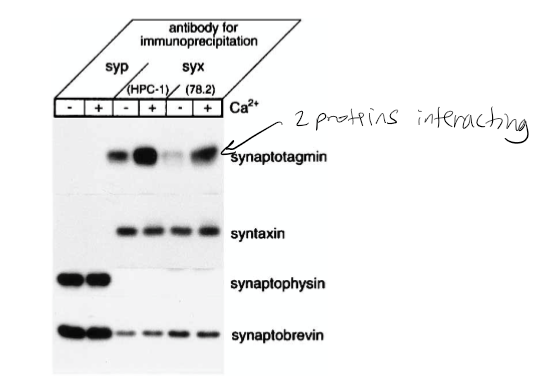
Figure 1: What were the controls?
Synaptobrevin and syntaxin. The controls in this experiment were syntaptophysin that was immunoprecipitated in parallel with monoclonal antibody Cl 7.3.
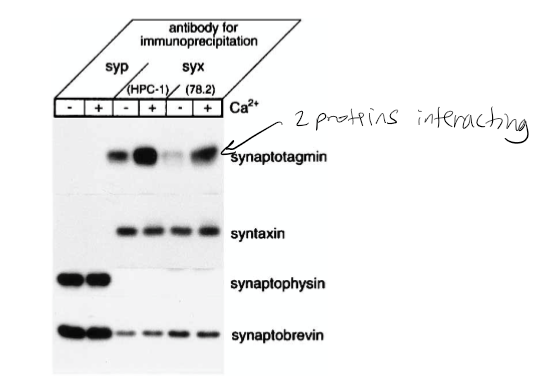
Figure 1: What do + and – indicate?
+ indicated presence of calcium and - indicates no calcium present in solution
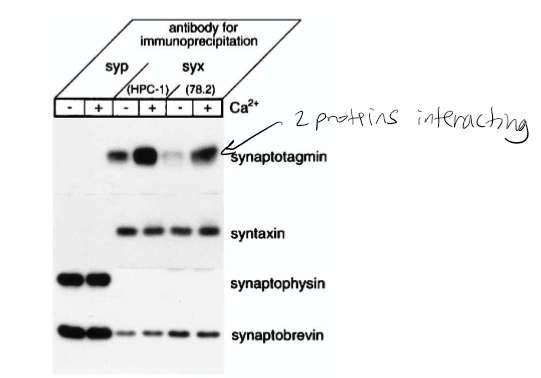
Figure 1: What is the conclusion and what part of the data led to this conclusion?
Syntaxin has calcium dependence with synaptotagmin. 1st line shown synaptotagmin calcium has bolder/darker line indicating calcium dependence. The conclusion of this was that the synaptophysin antibodies did not precipitate syntaxin 1 or the synaptotagmin. However, they did coprecipitate with synaptobrevin and synaptophysin. The Ca2+ increased the level of synaptotagmin associated with syntaxin 1. It was also found that the amount of synaptobrevin and SNAP-25 components for the SNARE complex that bound to syntaxin was not affected by Ca2+.
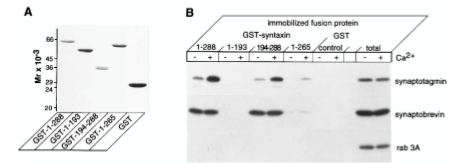
Figure 5: What experiment was conducted?
Where synaptotagmin binds on the syntaxin protein that it is trying to determine. The experiment conducted was to understand interactions between syntaxin 1 and synaptotagmin by using a Coomassie blue-stained gel of full-length and truncated forms of syntaxin 1 fused to GST. The proteins were visualized by staining with Coomassie blue to identify synaptotagmin binding.
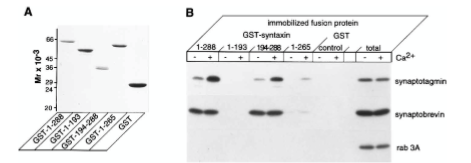
Figure 5: What are GST, GST-1-288, GST-1-193, etc.?
GST=carrier protein, GST-1-288= position of residue (position of amino acid full length of syntaxin. GST-1-193= position of amino acid, first half of syntaxin protein. 194-288 = refers to those amino acids. GST is a protein tag called glutathione-sepharose. GST-1-288 and GST-1-193 are residues of GST syntaxin. GST-1-288 residues result in a decrease in synaptotagmin binding activity. Synaptotagmin did not bind to the GST-1-193 residue of syntaxin 1 but did bind in a calcium-dependent way.
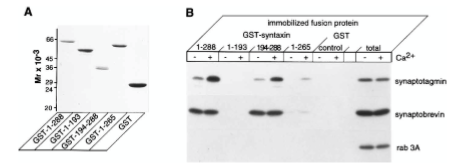
Figure 5: What were the controls? What does “total” indicate?
RAB 3A, synaptobrevin. 10 mg of synaptosomal extract.
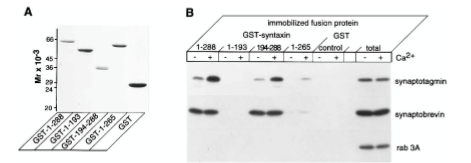
Figure 5: What do + and – indicate?
+ indicated presence of calcium and - indicates no calcium present in solution
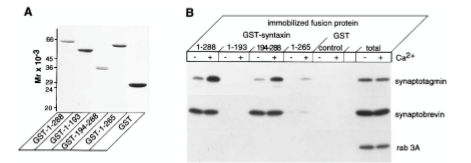
Figure 5: What is the conclusion and what part of the data led to this conclusion?
1-288 calcium has strong interaction on synaptotagmin. No bands on 1-193 syntaxin protein. Syntaxin 194-288 synaptotagmin binds in presence of calcium. 1-265 tiny bit of binding on syntaxin protein. Synaptotagmin binds to syntaxin protein between amino acid 265-288 or you can say 194-288. The conclusions of Figure 5 are that calcium regulates interactions between syntaxin 1 and synaptotagmin when there are no phospholipids present. The data that led to this was from the immunoblot analysis, which showed that immobilized full-length syntaxin 1 bound to synaptotagmin because the calcium did not modulate the amount of synaptobrevin bound to the immobilized syntaxin 1. It was also found that the removal of the transmembrane domain of the syntaxin caused a decrease in synaptotagmin binding activity, which caused a lowered ability of the syntaxin to bind to synaptobrevin.