Quiz 10: Pharmacology/Biomedical Sciences (Anderson)
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
90 Terms
What is the primary function of nuclear hormone receptors?
they regulate gene transcription by turning individual genes on or off depending on ligand binding
How does RNA synthesis relate to mRNA levels?
higher transcriptional rate → generally higher steady-state mRNA, unless modified by RNA degradation mechanisms
Why do different genes produce different amounts of mRNA?
each gene has a unique transcription rate, determined by promoter activity and epigenetic accessibility
How can the transcription rate of a single gene change?
hormones, developmental stage, and environmental signals can increase or decrease RNA synthesis for that gene
Why do hormones affect different genes differently?
only genes whose promoters contain specific hormone-response elements (HREs) respond to a given hormone
What defines a nuclear hormone receptor as a transcription factor?
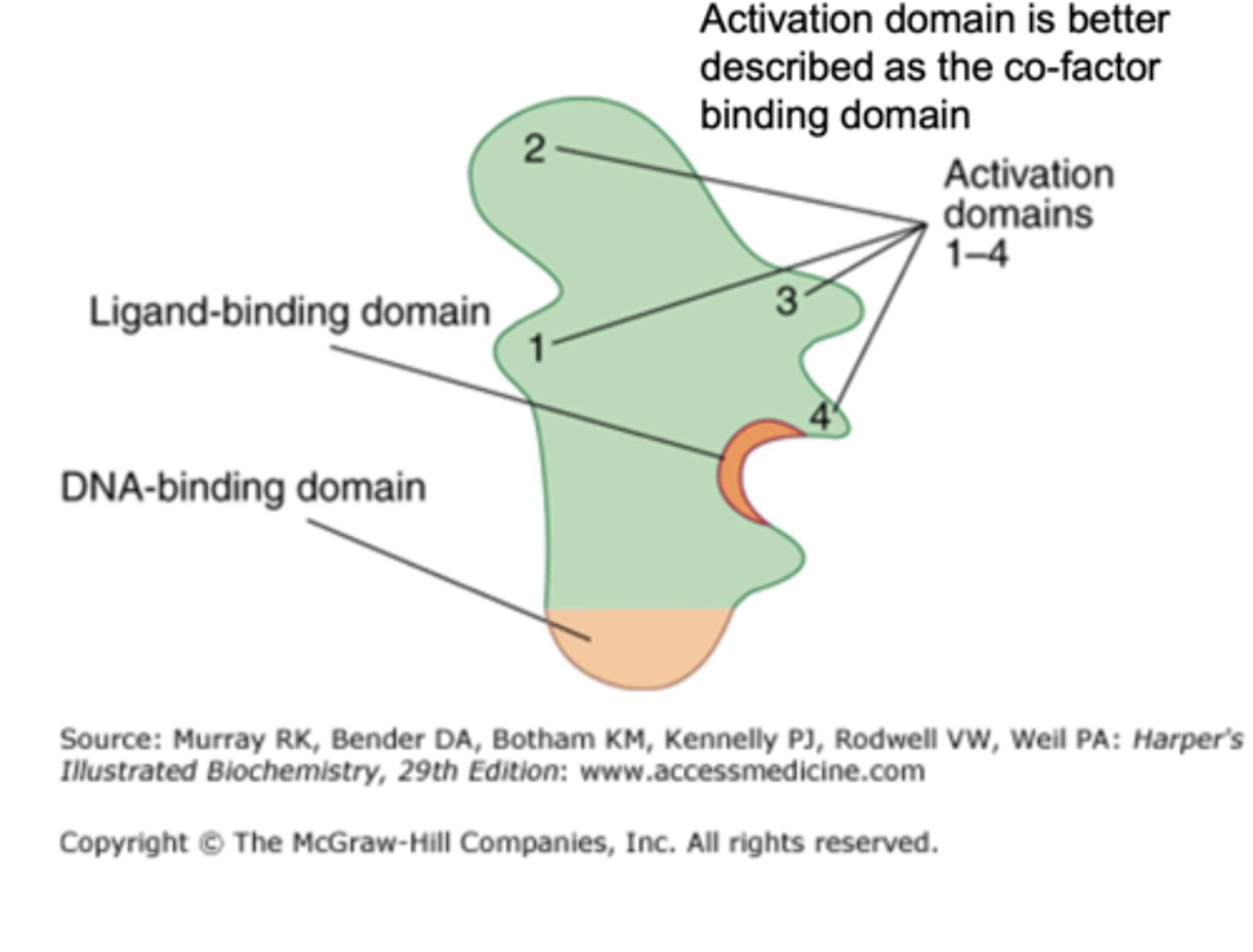
it contains a DNA-binding domain, ligand-binding domain, and activation (co-factor binding) domain
What are the three major functional domains of nuclear receptors?
DNA-binding domain, ligand-binding domain, and activation/co-factor-binding domain
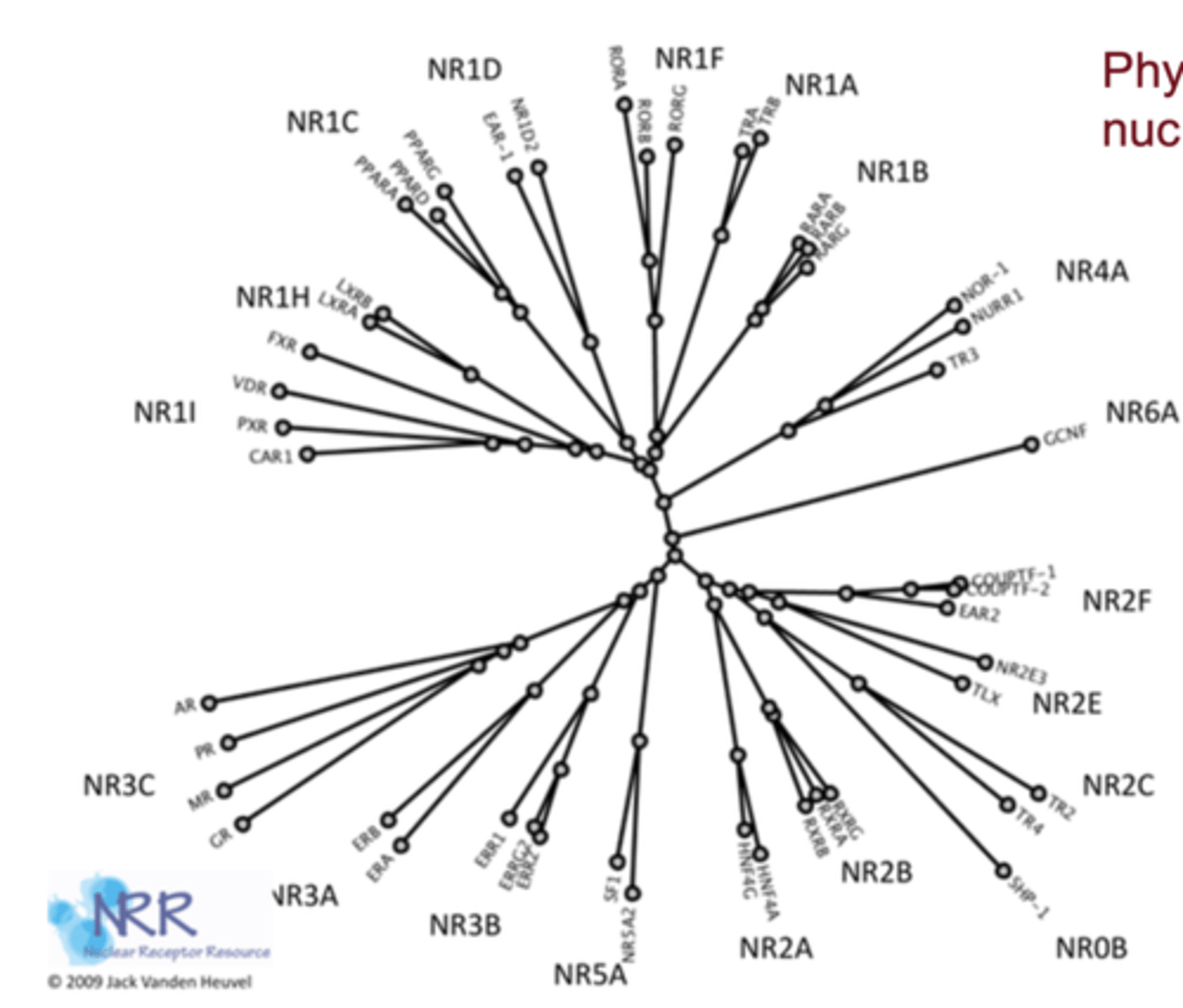
Why are nuclear receptors evolutionarily related?
their structures are conserved across species and likely derived from a common ancestral receptor gene
What determines ligand specificity for each nuclear receptor?
the structural chemistry of the receptor's ligand-binding pocket, which selectively fits only certain molecules
Why can some receptors (e.g., PPARs) bind multiple ligands?
they have a larger, more flexible binding pocket suited for fatty acids and structurally diverse drugs
Why do glucocorticoids exhibit mineralocorticoid activity?
cortisol/corticosterone structures closely resemble aldosterone, allowing them to bind the MR
Which enzymes normally protect MR from glucocorticoids?
11β-HSD2 converts cortisol → cortisone, which MR cannot bind
What happens when 11β-HSD2 is impaired or saturated?
cortisol binds MR, causing mineralocorticoid-like effects (HTN, sodium retention, edema)
What type of dimerization occurs with steroid hormone receptors?
they form homodimers in the cytoplasm and then move into the nucleus to bind DNA
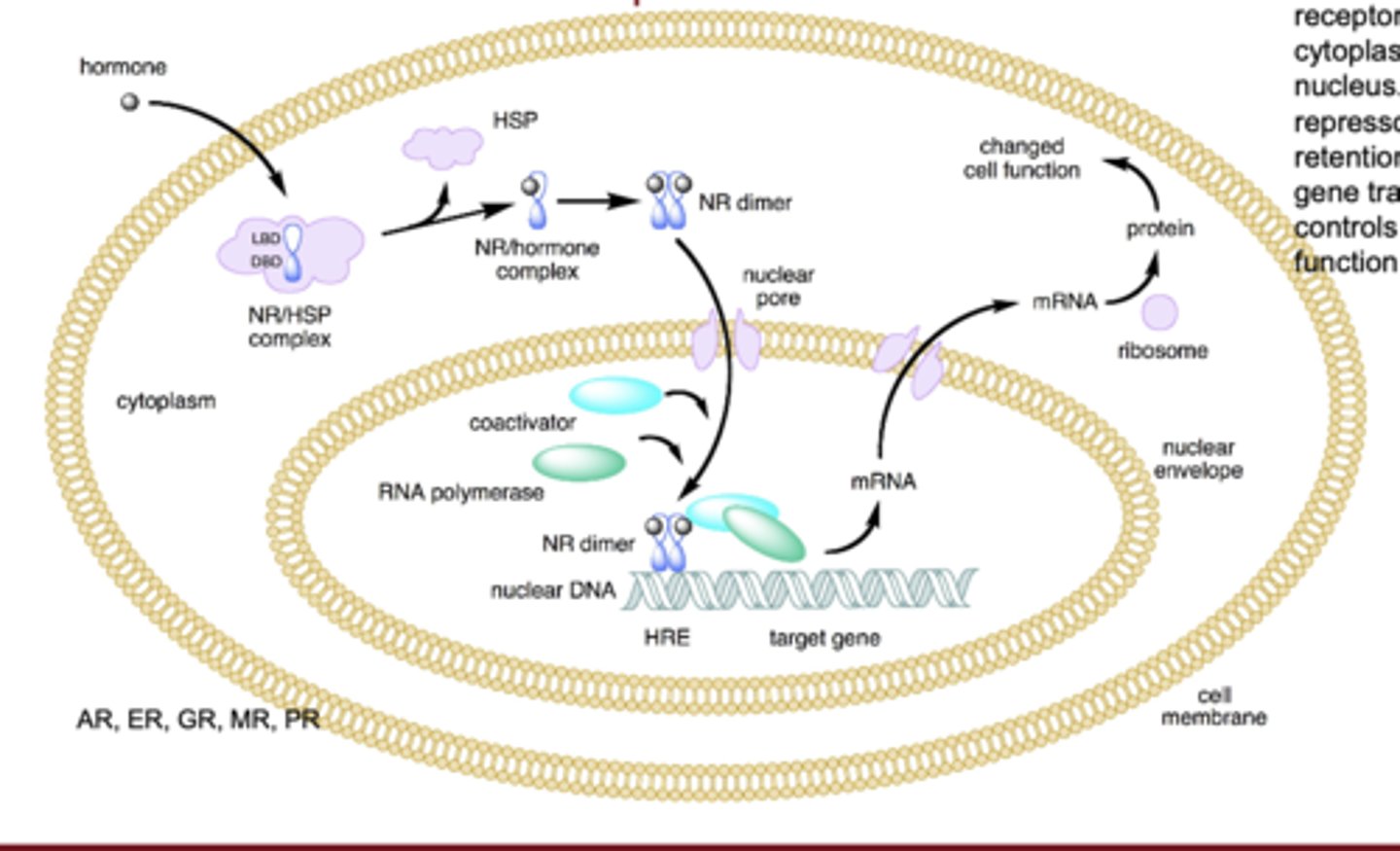
What type of dimerization occurs with non-steroid nuclear receptors?
they form heterodimers, commonly with RXR, inside the nucleus
Where are non-steroid nuclear receptors located before ligand binding?
txhey reside in the nucleus, already sitting on their response elements
What is a hormone-response element (HRE)?
a short DNA sequence in a gene promoter that recruits a specific nuclear receptor
What determines which tissues respond to a hormone?
only tissues whose chromatin is open at the relevant HRE-containing promoters can recruit the receptor
What is the role of co-repressors in nuclear receptor action?
they bind unliganded receptors and maintain chromatin in a closed/condensed state, preventing transcription
What is the role of co-activators in nuclear receptor action?
they bind ligand-activated receptors and open chromatin, allowing recruitment of transcription machinery
Which co-factor type typically acetylates histones?
co-activators, containing HAT (histone acetyltransferase) activity
What is the effect of histone acetylation?
neutralizes lysine's positive charge, reduces DNA-histone attraction, and opens chromatin (euchromatin)
Which co-factor type typically de-acetylates histones?
co-repressors, containing HDAC activity, remove acetyl groups and re-condense chromatin
What does an unliganded nuclear receptor recruit to maintain chromatin in a compact state?
it recruits HDAC-containing co-repressor complexes, which remove acetyl groups and keep chromatin condensed
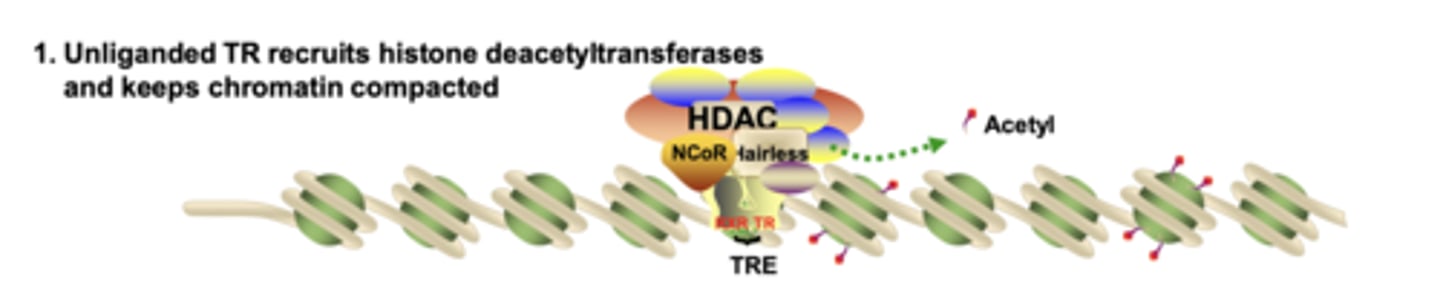
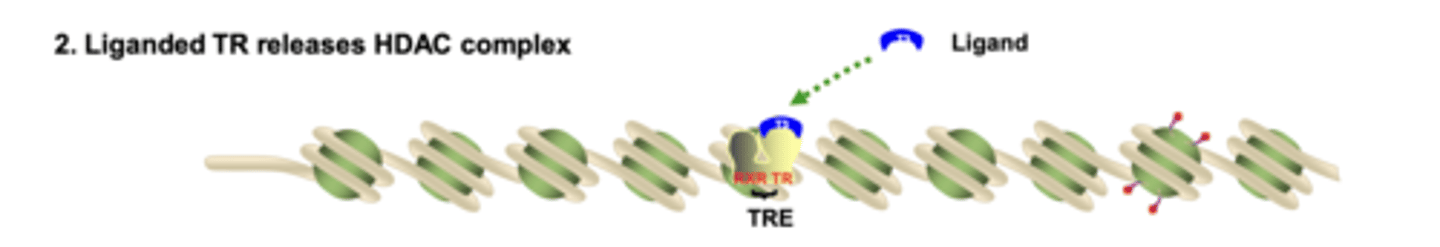
What happens to HDAC complexes when ligand binds the nuclear receptor?
ligand binding causes a conformational change that removes the HDAC co-repressor complex from the receptor
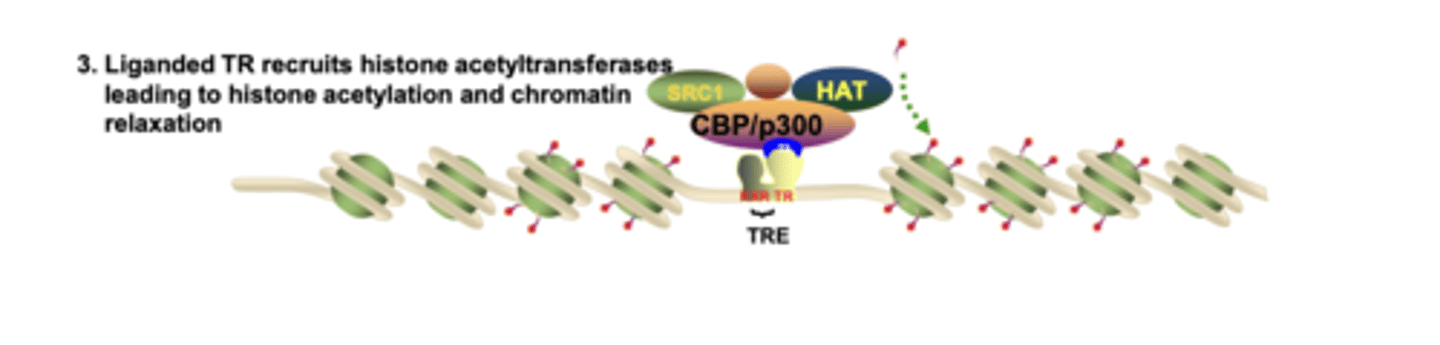
Which co-activator proteins are recruited by liganded nuclear receptors to acetylate histones?
SRC-1 and CBP/p300, which contain HAT activity, acetylating histones and opening chromatin
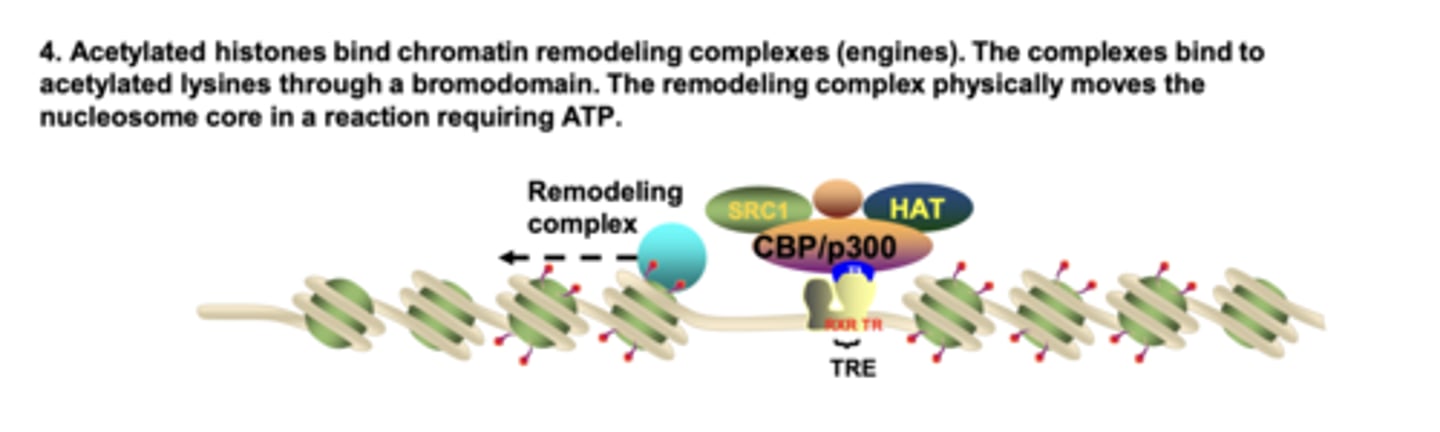
What binds to acetylated histone lysines, and what is its function?
chromatin remodeling complexes bind via bromodomains and use ATP to physically reposition nucleosomes
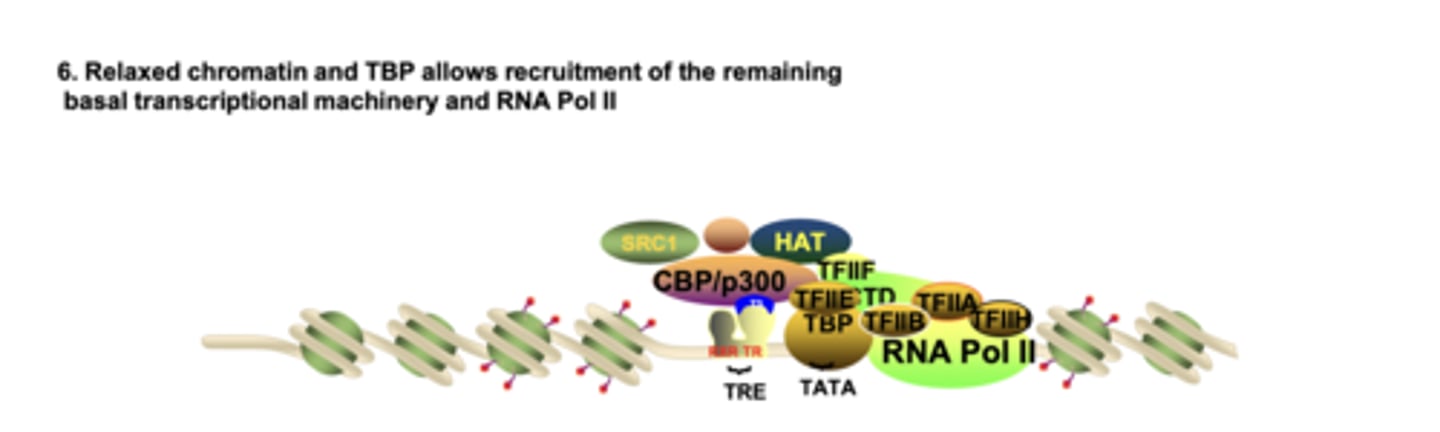
How do TAFs (TBP-associated factors) recognize the promoter when chromatin is relaxed?
TAFs contain bromodomains that bind acetylated lysines, enabling TBP recruitment to the TATA box

What allows the full basal transcription machinery and RNA Pol II to assemble at the promoter?
relaxed chromatin + TBP/TAFs + nucleosome repositioning expose the promoter for GTF and Pol II binding
What is euchromatin?
decondensed, accessible chromatin that allows for transcription factor binding and active gene expression
What is heterochromatin?
condensed, transcriptionally inactive chromatin with tightly packed nucleosomes
Which part of the gene defines the transcription start site?
the core promoter, including the TATA box, which binds TBP (TFIID)
What is the role of TBP (TFIID)?
it binds the TATA box, initiating assembly of general transcription factors (GTFs) required for Pol II
How do GTFs recruit RNA polymerase II?
GTFs assemble at the promoter and create a docking platform that precisely positions RNA Pol II at +1
How does transcription rate increase for a gene?
by recruiting more RNA Pol II per unit time or increasing Pol II elongation speed
How do nuclear receptors influence which genes can be transcribed?
they regulate chromatin accessibility at specific promoters, determining where GTFs can bind
Why does ligand binding change receptor behavior?
ligand binding causes a conformational change, switching co-repressor → co-activator association
What structural feature allows receptors to bind specific DNA sequences?
their zinc-finger DNA-binding domain, which recognizes unique HRE motifs
What is chromatin remodeling, and why is it essential?
the repositioning or modification of nucleosomes; required for Pol II and GTFs to access DNA
Why do nuclear receptor drugs mimic endogenous hormones so effectively?
because their pharmacologic activity uses the same biochemical pathways as endogenous signaling
Why is ligand entry into the nucleus an important pharmacologic consideration?
nuclear receptors function only inside the nucleus, so drugs must be capable of crossing membranes and accessing nuclear compartments
Where is the thyroid gland located anatomically?
it is located below the larynx and draped over the trachea in the neck
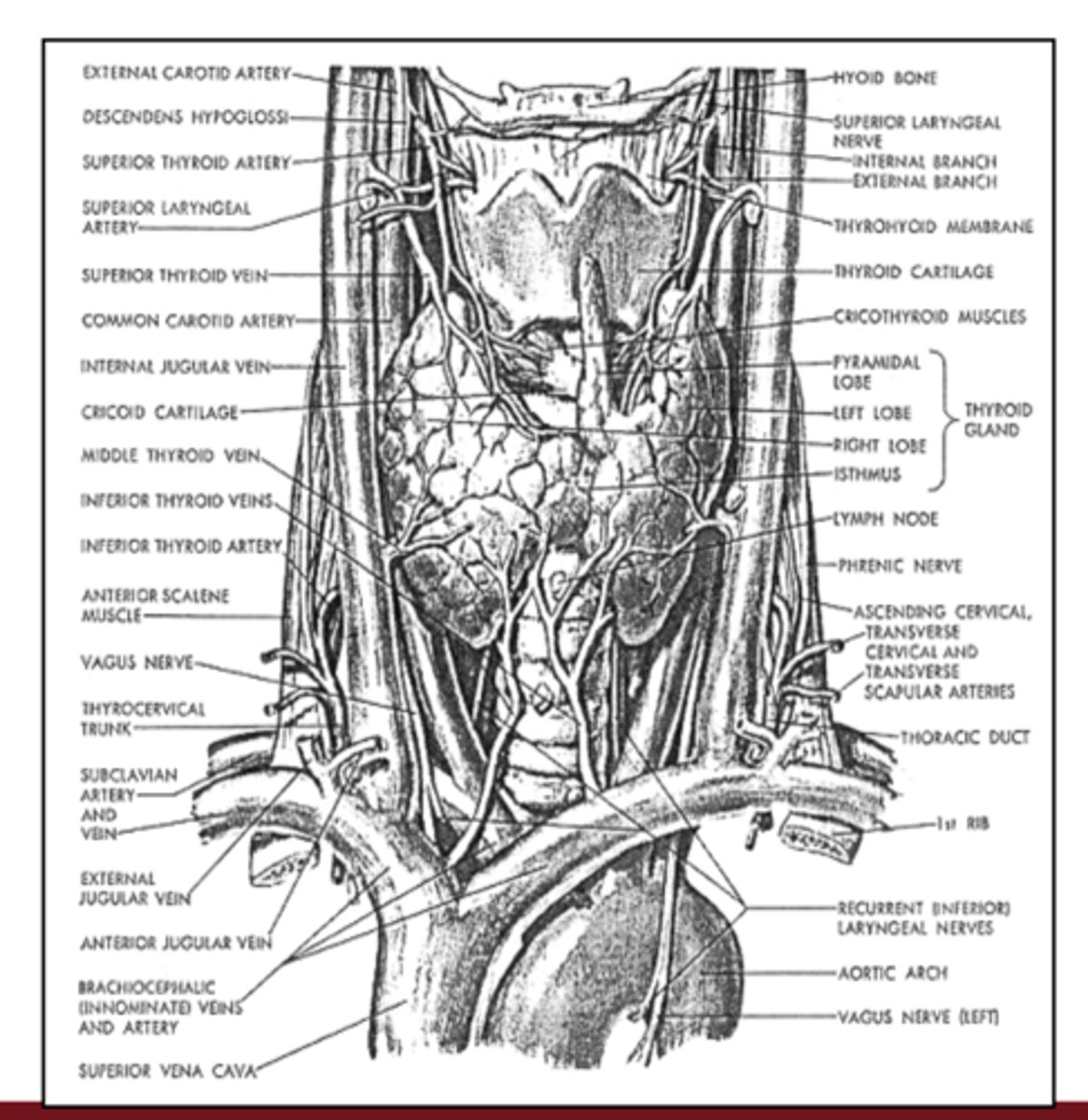
What is a goiter, and with which thyroid states can it be associated?
it is an enlargement of the thyroid gland and can occur in both hypothyroidism and hyperthyroidism
Why are iodine-deficient regions often mountainous or previously glaciated?
iodine comes from the ocean; regions far from the sea or heavily glaciated have low iodine in the soil, so crops and water provide less iodine
How much iodine is needed daily to avoid hypothyroidism and goiter?
about 100-200 µg of iodine per day
How is iodine sufficiency achieved at the population level?
by adding iodine to table and cooking salt, a safe and inexpensive public-health strategy
What basic building blocks are thyroid hormones derived from?
two tyrosine residues joined by an ether linkage with iodine incorporated; T4 has four iodines, T4 has three
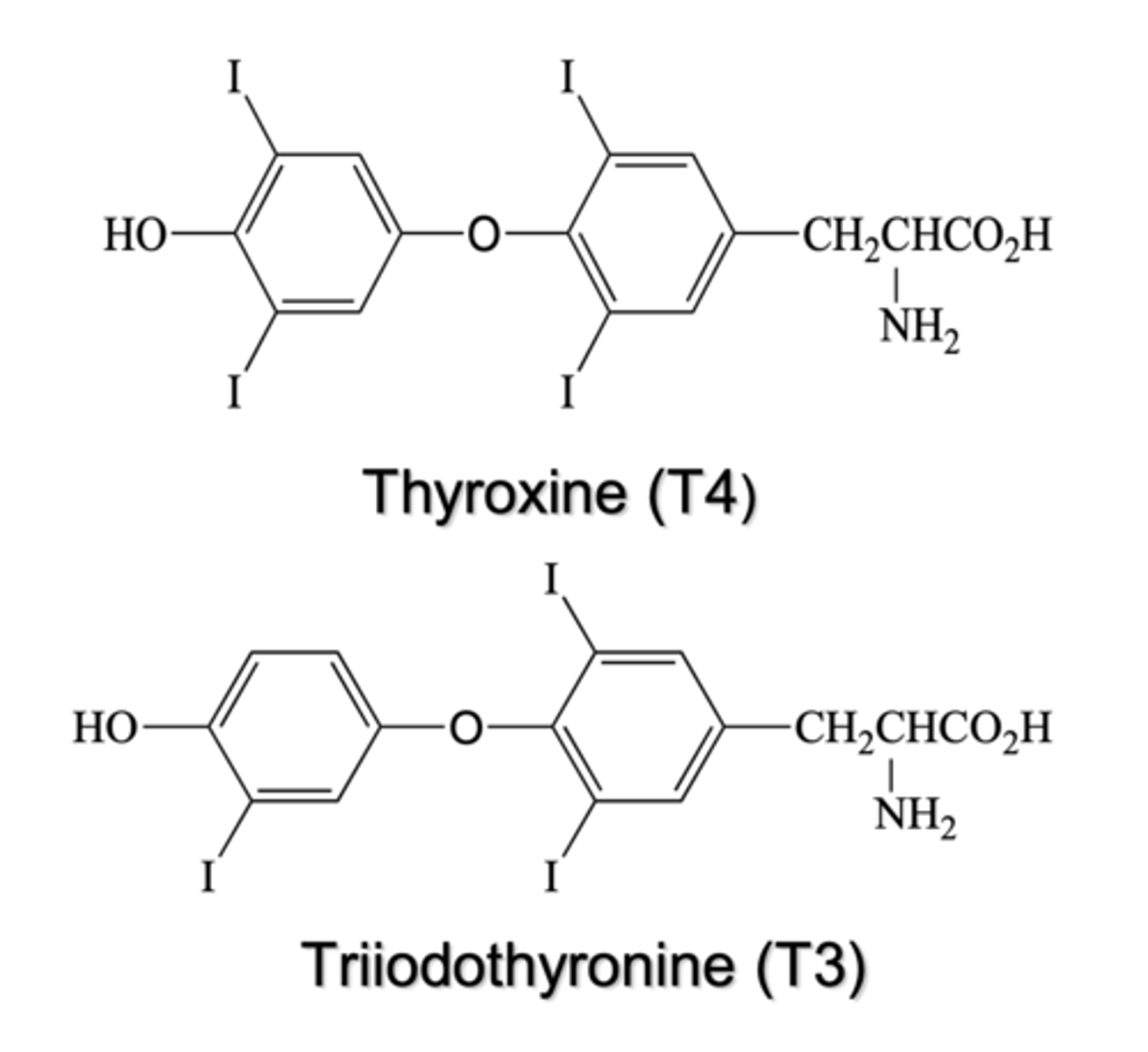
Why are thyroid hormones clinically important from a prescribing perspective?
they are the most commonly prescribed drugs in the U.S. (ex. levothyroxine)
How common are hypo- and hyperthyroidism in the population, and in which sex are they more prevalent?
hypothyroidism is more common, and both conditions occur more often in females due to higher autoimmune disease risk
Which tissue produces TRH, and what is its role in the HPT axis?
the hypothalamus produces TRH (thyrotropin-releasing hormone), which stimulates the pituitary to release TSH
Which tissue produces TSH, and what does TSH do?
the anterior pituitary secretes TSH (thyroid-stimulating hormone), which stimulates the thyroid gland to synthesize and release T4 and T3
How do T4 and T3 regulate the HPT axis by feedback?
circulating T4/T3 put negative feedback on both the pituitary (TSH) and hypothalamus (TRH), reducing further hormone release when levels are enough or high
What is the overall function of the hypothalamic-pituitary-thyroid (HPT) axis?
to maintain appropriate circulating thyroid hormone levels through a classic negative-feedback loop involving hypothalamus, pituitary, and thyroid
Which transporter brings iodide from blood into the thyroid follicular cell, and how is it regulated?
sodium-iodide symporter (NIS) transports iodide into thyrocytes; its activity is stimulated by TSH
What enzyme organifies iodine and where is it located?
Thyroid peroxidase (TPO); found on the apical surface of thyrocytes, adds iodine to tyrosines within thyroglobulin
What are MIT and DIT?
MIT = monoiodotyrosine (one iodine on tyrosine)
DIT = diiodotyrosine (two iodines)
How are MIT/DIT formed?
generated when TPO organifies iodide onto tyrosyl residues in thyroglobulin
What is meant by "organification" in thyroid hormone synthesis?
the process by which iodine is covalently attached to tyrosines in thyroglobulin, converting them into MIT and DIT
What is the coupling reaction in thyroid hormone synthesis, and which enzyme catalyzes it?
coupling is the joining of iodinated tyrosines via an ether linkage to form T4 (DIT+DIT) or T3 (MIT+DIT); it is also catalyzed by TPO
How does iodine availability influence T4 vs T3 production?
Adequate iodine: more DIT is formed and more T4 is produced
Iodine deficiency: proportionally more MIT/T3 is produced and the T4:T3 ratio falls
How are T4 and T3 released from thyroglobulin into the bloodstream?
thyroglobulin is endocytosed and proteolyzed inside thyrocytes; proteases cleave off T4 and T3, which then diffuse into the blood
In the blood, how are most thyroid hormones carried?
they are tightly bound to plasma proteins such as thyroxine-binding globulin (TBG), transthyretin, and albumin; only small free fraction is biologically active
What is the main circulating form vs the main active form of thyroid hormone?
- T4 is the major circulating prohormone
- T3 is the more potent active hormone at tissue receptors
What enzymes convert T4 to T3 in peripheral tissues, and why is this important?
deiodinases (type 1 and type 2) remove an outer-ring iodine from T4 to produce active T3, allowing tissue-specific regulation of thyroid hormone activity
Which deiodinase inactivates thyroid hormone, and how?
Type 3 deiodinase removes an inner-ring iodine, converting T4 to reverse T3 (rT3) and T3 to T2, thereby inactivating hormone
At the cellular level where do thyroid hormones primarily act, and through what receptor?
they act in the nucleus by binding thyroid hormone receptors (TR), which are nuclear receptors that regulate gene transcription at thyroid response elements
How does T3 binding affect TR/RXR complexes at DNA?
the complex releases co-repressors, recruits co-activators, remodels chromatin, and increases or decreases transcription of target genes
What are some major physiologic roles of thyroid hormones in metabolism?
increase basal metabolic rate, stimulate oxygen consumption, enhance carbohydrate and lipid metabolism, and promote heat production (thermogenesis)
How do thyroid hormones affect the cardiovascular system?
increase heart rate and contractility, up-regulate β-adrenergic receptors, and contribute to vasodilation and increased cardiac output
What are key neuromuscular/CNS effects of thyroid hormones?
important for normal brain development and, in adults, influence alertness, reflexes, mood, and neuromuscular function
What are typical clinical features of hypothyroidism?
fatigue, weight gain, cold intolerance, constipation, bradycardia, dry/coarse skin, and sometimes myxedematous thickening of skin and facial features
What are typical clinical features of hyperthyroidism?
weight loss (or difficulty gaining), heat intolerance, tachycardia/palpitations, warm moist skin, increased GI motility with frequent soft stools, tremor, irritability, and often a goiter
What is Graves' disease and how does it cause hyperthyroidism?
autoimmune disease where TSH-receptor-stimulating IgG antibodies drive excessive thyroid hormone production
What are other major causes of hyperthyroidism?
toxic adenoma, toxic multinodular goiter, thyroiditis (initial hyper phase), exogenous T4/T3 ingestion (ex. hamburger toxicosis), and iodine/amiodarone-induced disease
How does thyroiditis lead to a transient hyperthyroid phase?
it causes release of preformed thyroid hormone, producing temporary hyperthyroidism that can later progress to hypothyroidism as hormone stores are depleted
What is a common cause of primary hypothyroidism in iodine-sufficient regions?
autoimmune thyroiditis (Hashimoto's)
Which lab values are most clinically useful for assessing thyroid status?
serum TSH (most sensitive), free T4, and sometimes free T3; autoantibodies (ex., TPOAb, TSH-receptor antibodies)
What lab pattern suggests primary hypothyroidism?
high TSH with low free T4 (and often low/normal T3)
What lab pattern suggests primary hyperthyroidism (like Graves' disease)?
low or undetectable TSH with high free T4 and/or T3
What is the basic principle of hypothyroidism treatment?
replace the missing hormone, usually with synthetic T4 (levothyroxine), and titrate to achieve a normal TSH and free T4
What are the advantages of levothyroxine (T4) as thyroid replacement?
stable, uniform content, low cost, long half-life, easy serum level monitoring, converted to T₃ in tissues
When might liothyronine (T4) be used clinically?
since it is 3-4× more potent with rapid onset and short duration, T₃ is reserved for situations needing rapid action, such as myxedema coma or when pts don't feels well on T4 alone
What is a T4/T3 combination product?
preparation containing T₄ and T₃ in about a 4:1 ratio to mimic physiology; not usually necessary
What are the main treatment options for hyperthyroidism?
radioactive iodine ablation (I-131), anti-thyroid drugs, and surgery (thyroidectomy)
What is the major downside of radioactive iodine therapy?
radioablation often results in permanent hypothyroidism, requiring lifelong thyroid hormone replacement
How do anti-thyroid drugs work?
they block iodine uptake, deiodination, hormone release, or TPO activity, thereby reducing synthesis or release of thyroid hormones.
What is the primary mechanism of thionamides (ex. methimazole, PTU)?
inactivate thyroid peroxidase, inhibiting iodination (organification) and coupling reactions
In which life-threatening condition are high-dose iodide and PTU particularly useful, and why?
a thyroid storm
- PTU rapidly blocks new synthesis and T4→T3 conversion, and large iodide doses acutely inhibit hormone synthesis and release
How does Lugol's solution (strong iodine) act as an antithyroid treatment?
high concentrations of iodide transiently inhibit thyroid hormone synthesis and release; effect is rapid (within ~24 h) but escapes after 7-14 days (short-term)
Which older inorganic anion drugs inhibit iodide uptake?
thiocyanate and perchlorate competitively inhibit iodide uptake via NIS but are no longer used in the U.S. due to toxicity