Enzymes
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
Activation energy
Initial energy input in a reaction
Enzyme
Proteins
3D shape
intricate chemical structure
depends on weak interactions between amino acids of the protein
Biological catalysts
Substrates > Enzymes > Products
Exergonic reaction
Occur when the products of a chemical reaction have less energy than the reactant, tend to be catabolic
Endergonic reaction
Occurs when the products of a chemical reaction have more energy than the reactants - tend to be anabolic
Metabolism
Complex network of interdependent and interacting chemical reactions in living organisms
most are intercellular
form pathways wherein 1 type of molecule is transformed into another by small steps
Substrate
Starting substance in an enzyme-catalyzed reaction
Catalyst
Increases the rate of a chemical reaction, however is not changed by a chemical reaction
don’t get used up by chemical reactions, so can catalyze many reactions
__________________ increases the chance a collision between a substrate and active site will occur - catalysis
Higher kinetic energy
Extracellular enzymes
Enzymes exported from cells placed in vesicles and secreted by endocytosis to work externally
Intercellular enzymes
Non-secreted enzymes
Enzyme Specificity (& its benefits)
each one catalyzes a specific reaction, or a specific group of reactions
Because of enzyme specificity, organisms have to make large numbers of different organisms
BENEFITS:
allows organisms to control metabolism
a cell produces a particular enzyme which causes a specific reaction
the rate of the reaction can be controlled by producing more or less of an enzyme
mechanisms do exist for stopping certain enzymes from working if they aren’t needed
Anabolic Reaction
Small molecules > large molecules
require energy
e.g. photosynthesis
uses Co2, H2O and more to make larger molecules with energy from light
macromolecules can be formed from monomers using ATP
this is a condensation reaction
EXAMPLES:
protein synthesis (translation) by ribosomes
DNA synthesis (replication)
Synthesis of complex carbohydrates like starch, cellulose, and glycogen
Catabolic reaction
Large > Small
release energy
that energy can be captured by coupling the catabolic reaction to the synthesis of ATP
EXAMPLES:
digestion of food
cell respiration
digestion of complex carbon compounds
e.g. decomposers
Enzyme-substrate binding
For catalysis, substrates must bind to the active site
the shape and chemical properties of the substrate and active site match
therefore a certain substrate can bond while others cannot
active sites vary in size depending on substrate size
usually a few amino acids at the active site are essential to create chemical conditions that change substrates enough to convert them to products
the amino acids which form the active site are brought together by folding polypeptides
Steps to enzyme-substrate reaction
Substrate approaches active site
until it nears the enzyme, its direction of movement is random
once it’s close enough, the chemical properties of the enzyme surface attract the substrate towards the active site
Substrate binds to the active site
3D molecular shapes change (induced-fit binding)
If there is a second substrate, it approaches and binds to another part of the active site
the substrate and active site cause changes in each other to allow for binding
changes to substrates make it easier for bonds to break and new bonds to form
Products (once converted) detach, active site returns to its original state and the catalytic cycle repeats
Molecular motion in liquids
molecules are packed closely together but are free to move
direction of each molecule changes rapidly and at random
if a liquid has substrates and enzymes, they can come together
this rate will be higher if:
there is a high concentration of substrates or enzymes
or if temperature increases, leading to faster molecular motion
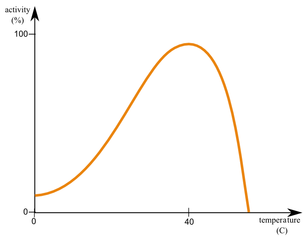
Effects of temperature on rate of enzyme activity
when liquid is heated, particles gain kinetic energy, and therefore move more quickly, and the chance of a substrate colliding with an active site increases, increasing enzyme activity
when liquid is too heated, bonds vibrate more and are more prone to breakage, so the structure of enzymes change and they are often denatured
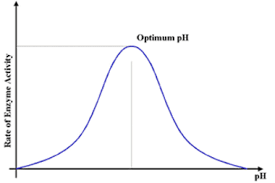
Effects of pH on enzyme activity
enzymes are sensitive to chemical environment
most enzymes have an optimum pH which is where they have the most activity
if pH increases or decreases, bonds between amino acids are altered, changing the structure so that they can’t bind with substrates
beyond a certain pH, they are irreversibly denatured
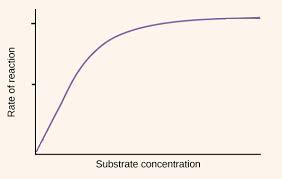
Effects of substrate on enzyme activity
when the substrate concentration increases, substrate-active site collisions occur more frequently and enzyme catalysis reaction rates will increase
however, as substrate conversation increases, more and more active sites are occupied, meaning that a greater proportion of substrate-active sites are blocked
the increases in enzyme catalase reaction rates get smaller
Independent, dependent and control variables (enzyme activity)
INDEPENDENT:
temperature
substrate concentration
enzyme concentration
pH
DEPENDENT:
reaction rate
enzyme activity
CONTROL:
all factors that affect enzyme activity other than independent
Reaction Rate:
speed at which substrates are converted to products
How to measure enzyme activity
Allow reaction to occur for a fixed time, and then count the amount of substrate that is used up, and the amount of product that is formed
start with a known amount of substrate and allow the reaction to continue until all substrates are converted to products
Exothermic reaction
a reaction without an enzyme that has a net RELEASE of energy