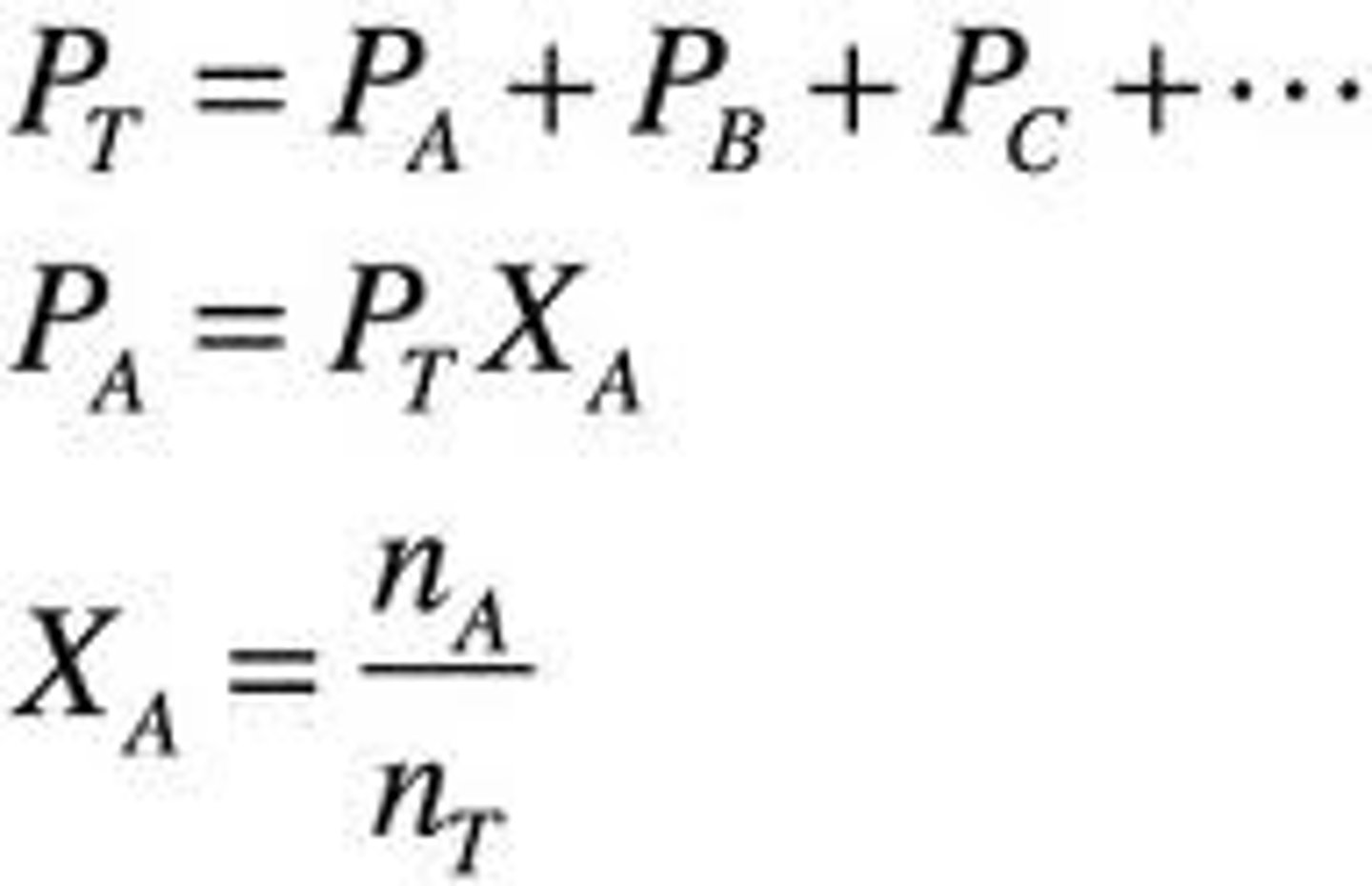Inorganic Chemistry
1/169
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
170 Terms
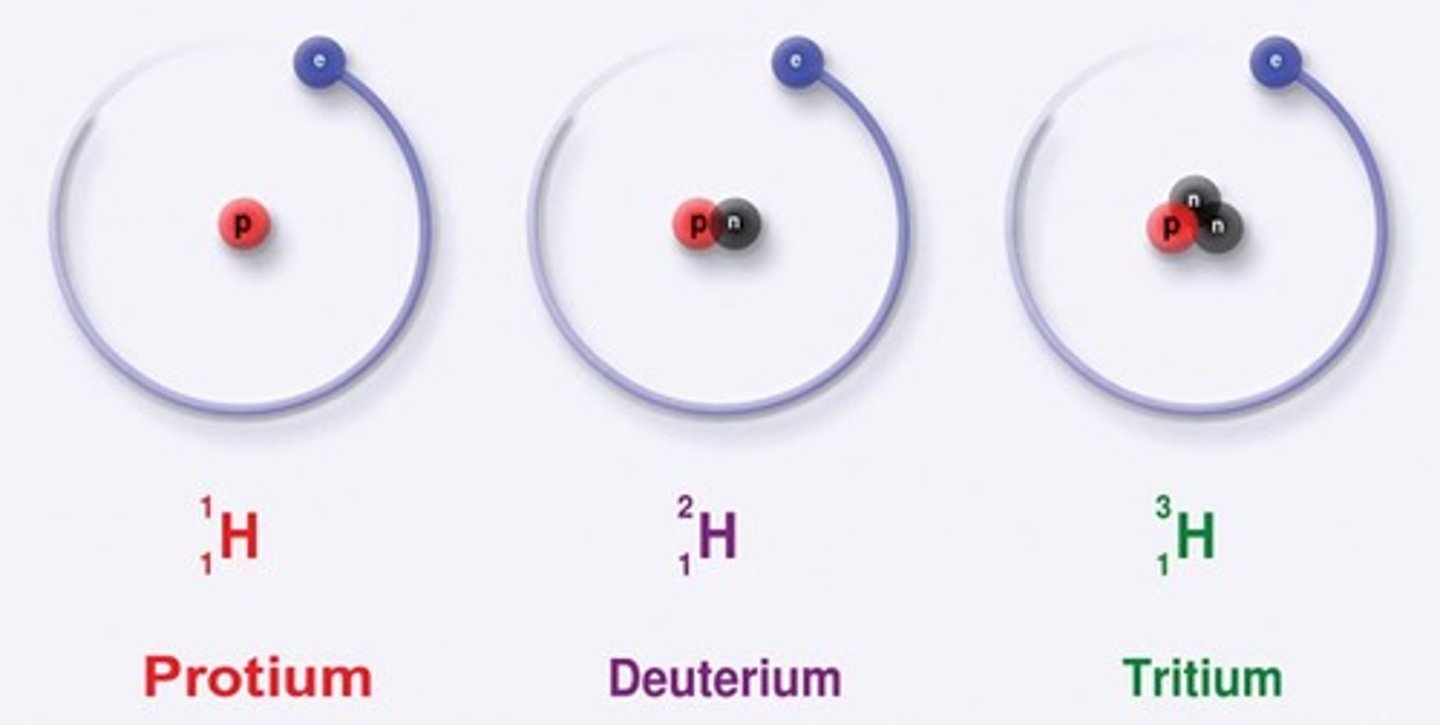
Various Isotopes of Hydrogen
Atoms of the same element have the same atomic number (Z= 1), but may have varying mass numbers (A= 1, 2, 3,)
Atomic Number
- Number of Protons
- Number of Protons = Number of Electrons (in a neutral atom)
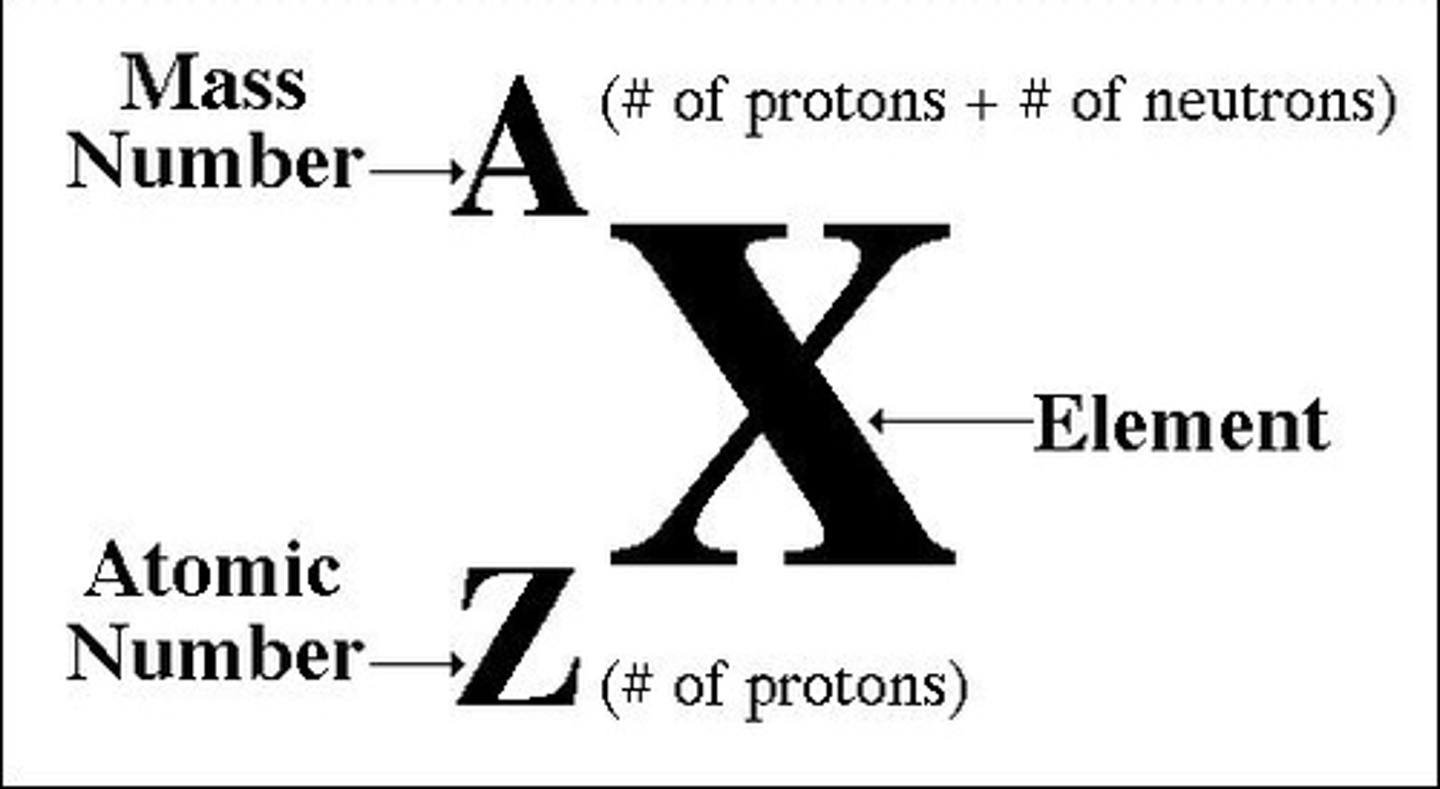
Atomic Mass Number
- Protons + Neutrons
- Electrons are not included in mass calculations because they are much smaller. (1/2000) that of a proton.
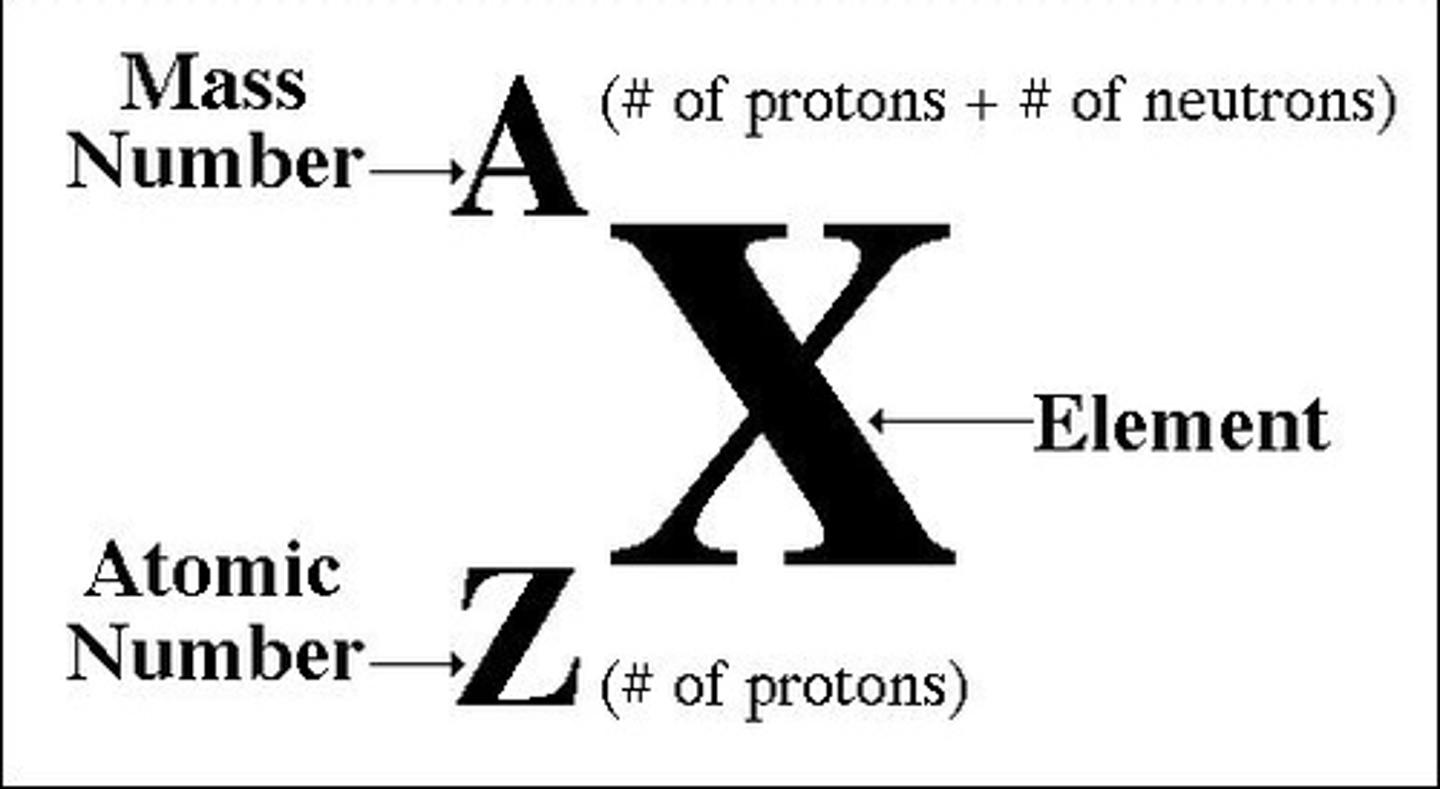
Atomic Weight
Weighted average of the naturally occurring isotopes of an element.
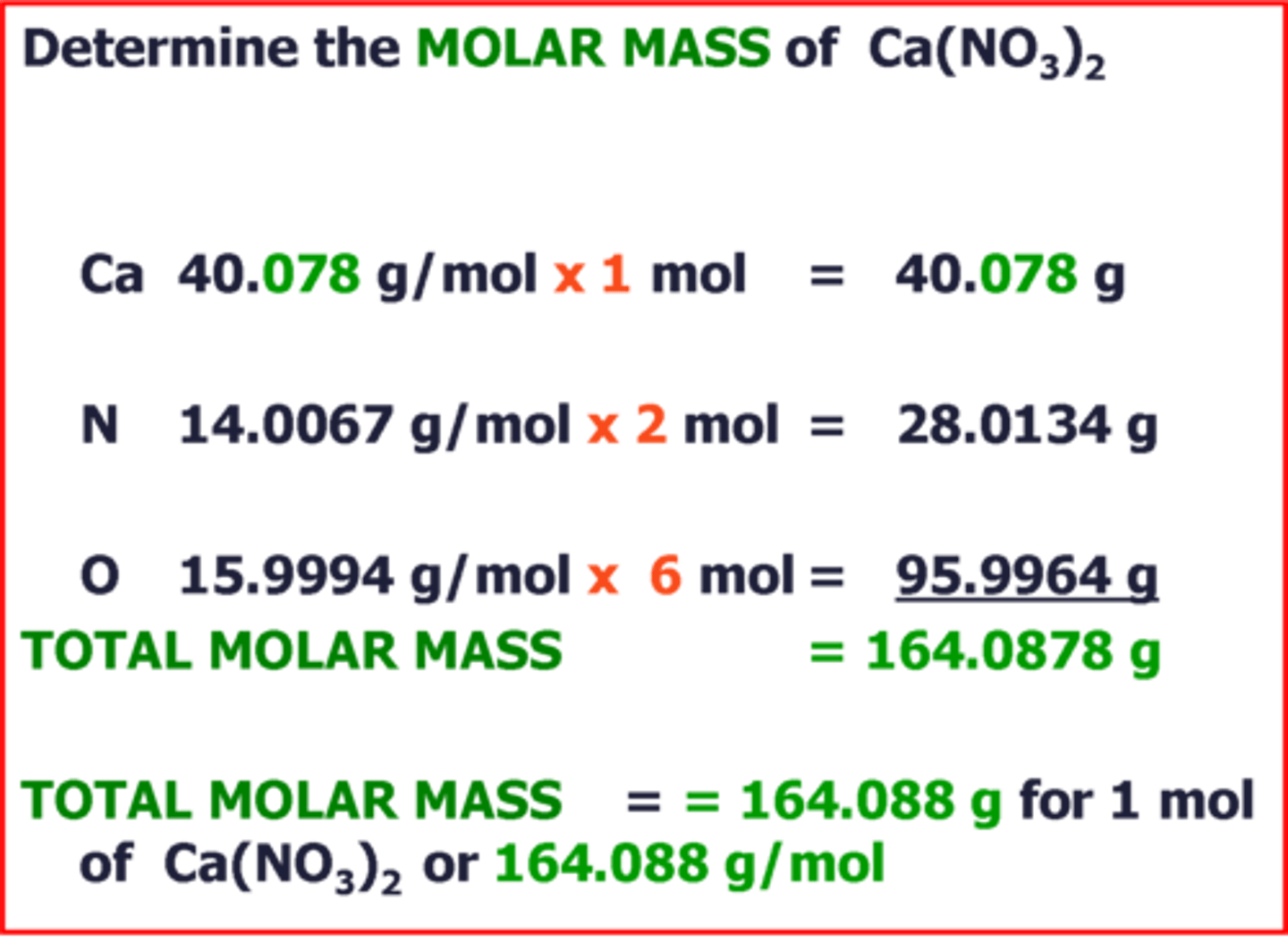
Molar Mass
Grams per mol (g/mol)
Planck Relation
- Quanta: Energy emitted as electromagnetic radiation from matter comes in discrete bundles.
- E = hf
- h= 6.62610^-34 Js
- Energy is inversely proportional to wavelength
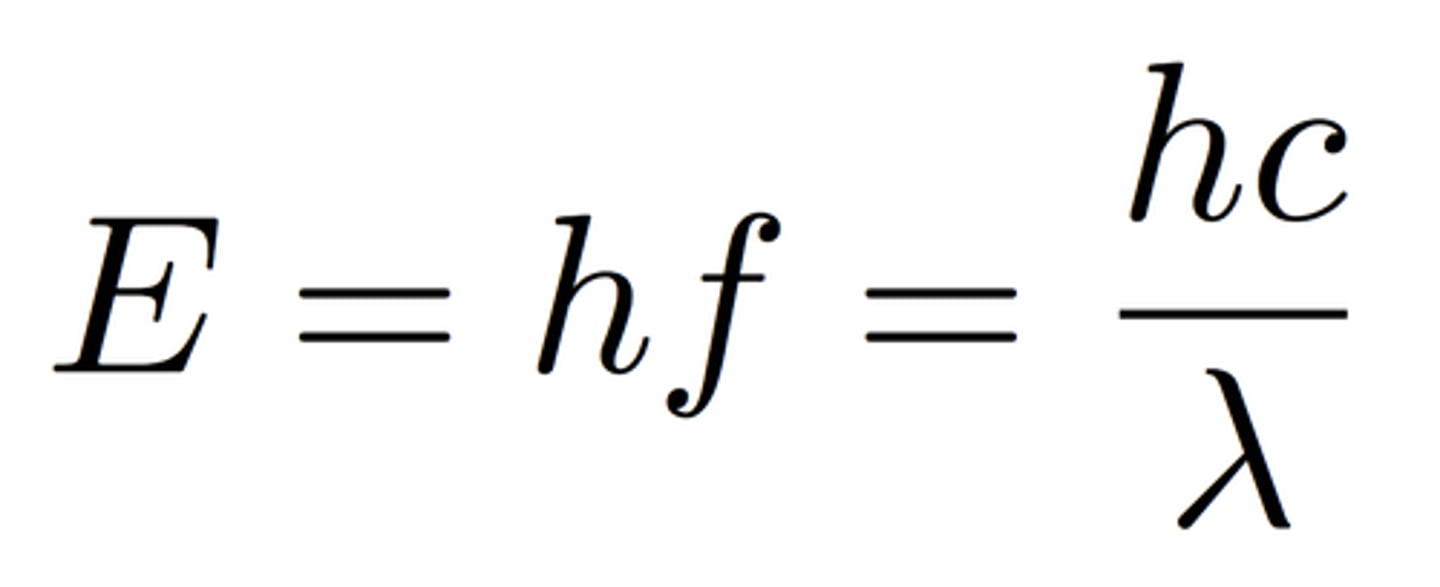
Speed of Light
c= 3 * 10^8 m/s

Angular Momentum of an electron orbiting
- n is the principal quantum number
- h is planck's constant = 6.62610^-34 Js
- Part of Bohr Model
- L = n-1
Energy of the electron
- E is the energy of the electron
- RH: Rydberg unit of energy; 2.18*10^-18 J/electron
- n: Principal quantum number
- Due to the negative sign, (E) is directly proportional to the principal quantum number (n)
- Positive E: Emission of energy (electrons move to orbits of lower energy)
- Negative E: Absorption (color we see is the light that is not absorbed by the compound)
- Part of Bohr Model
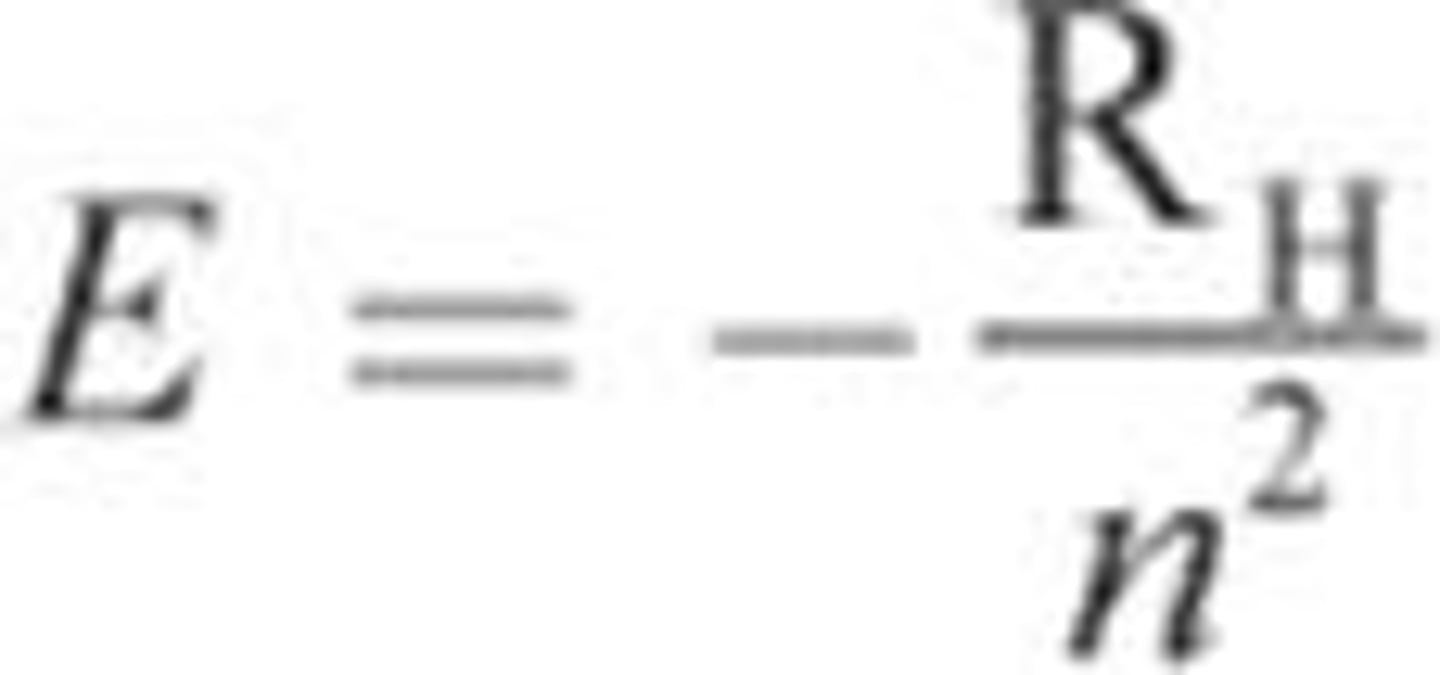
Ground State of an atom
- State of lowest energy (n=1), in which electrons are in the lowest possible orbitals.
- Bohr's Nobel prize winning model
Heisenberg uncertainty principle
It is impossible to simultaneously determine, with perfect accuracy, the momentum and the position of an electron
Bohr model of the atom
A dense positively charged nucleus is surrounded by electrons revolving around the nucleus in orbits with distinct energy levels.
Quantum
- Energy difference between energy levels. First described by Planck.
- Electrons can only exist at certain energy levels.
- The energy of an electron increases the further it is from the nucleus.
Atomic Absorption Spectrum
- For each element is unique
- For an element to jump from a lower energy to a higher one, it must absorb an amount of energy precisely equal to the energy difference between the two levels.
Atomic Emission Spectrum
- When electrons return from the excited state to the ground state, they emit an amount of energy that is exactly equal to the energy difference between the two levels.
Quantum Mechanical Model
- Electrons do not travel in defined orbits, but rather localized in orbitals
- Orbitals: A region of space around the nucleus defined by the probability of finding an electron in that region of space.
Heisenberg Uncertainty Principle
- States that is it is impossible to know both an electron's position and its momentum exactly at the same time.
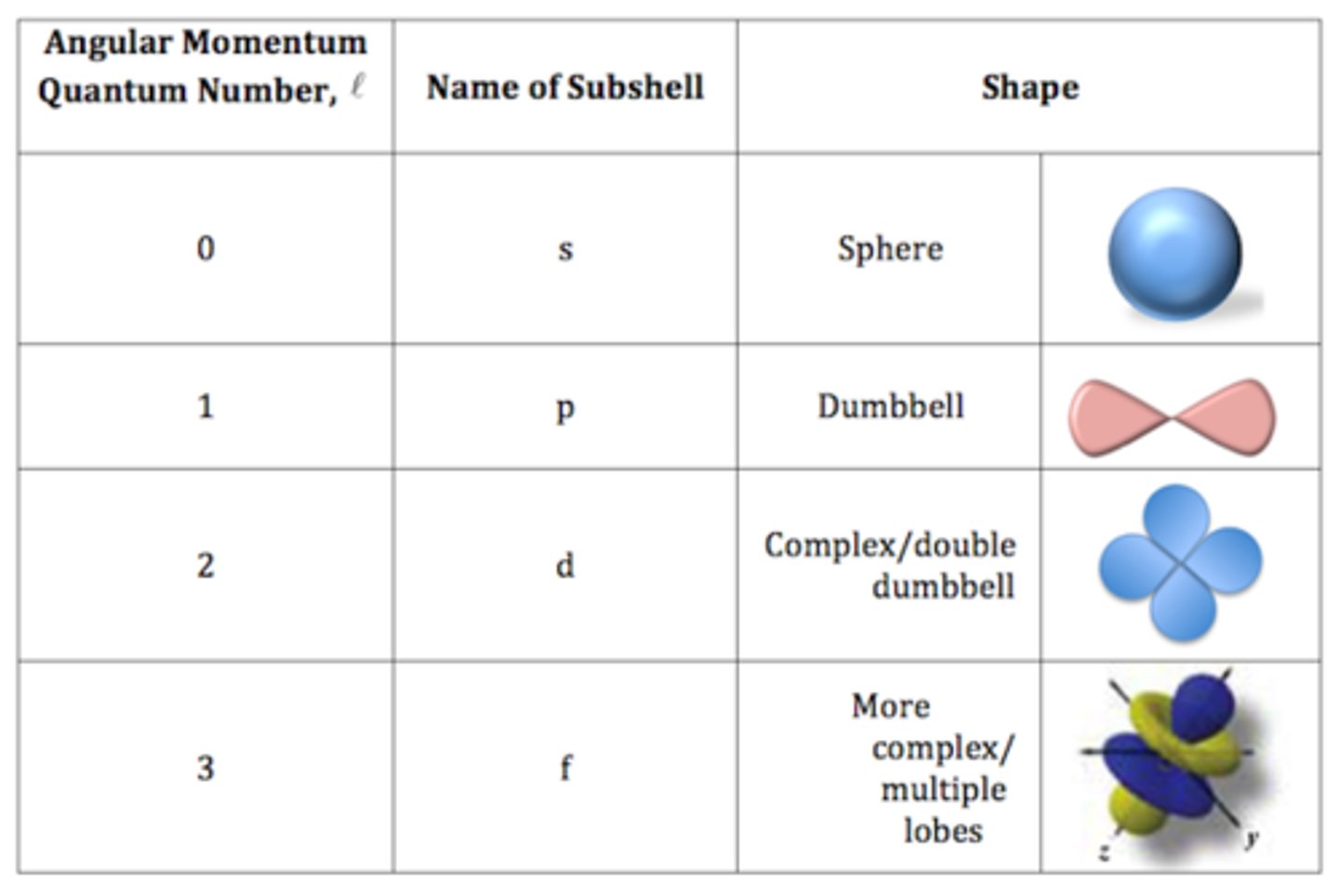
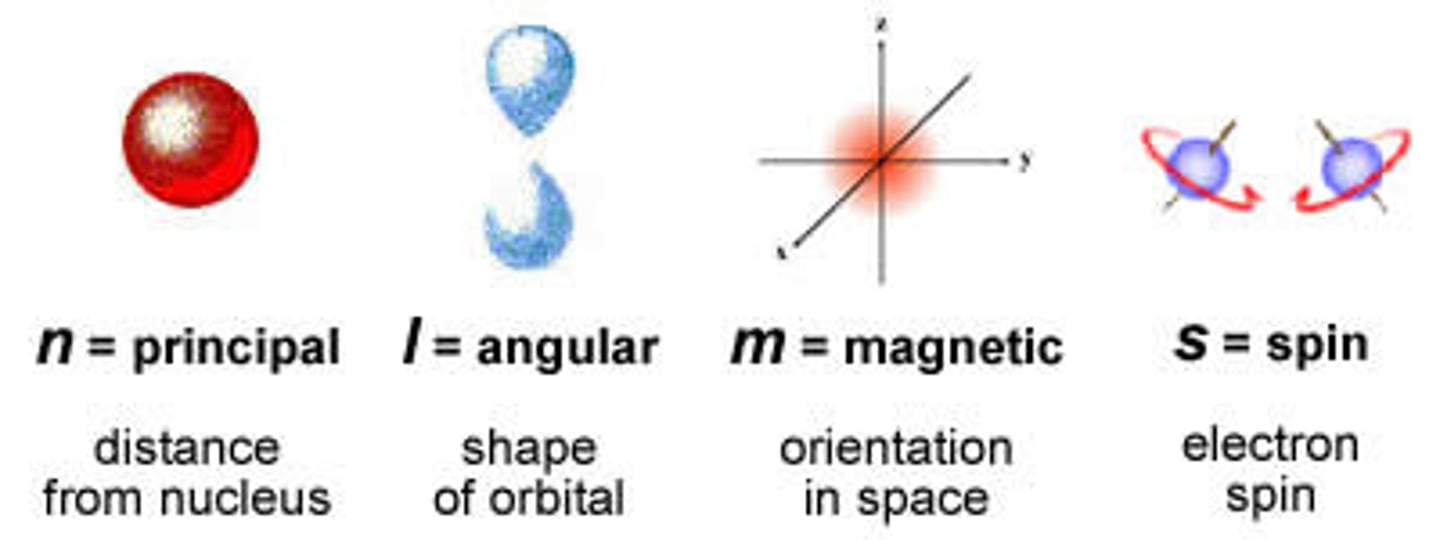
Quantum Numbers
- Describe any electron in an atom
- Principal Quantum number, n: average energy of a shell.
- Azimuthal quantum number, l: describes sub shells within a given principal energy level (s, p, d, f)
- Magnetic Quantum number, ml: Specifies the particular orbital within a subshell where an electron is likely to be found.
- Spin Quantum number, ms: indicates the spin orientation (+/- 1/2)
n + l rule
- Electrons fill the principal energy levels and subshells according to increasing energy level.
Hund's Rule
- States that subshells with multiple orbitals (p, d, f) fill electrons so that every orbital in a subshell gets one electron before any of them gets a second one.
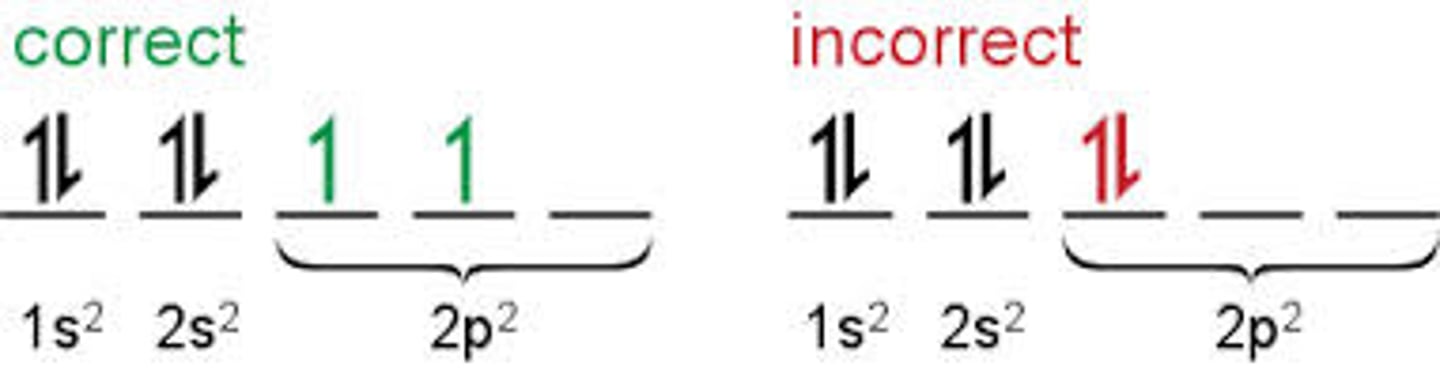
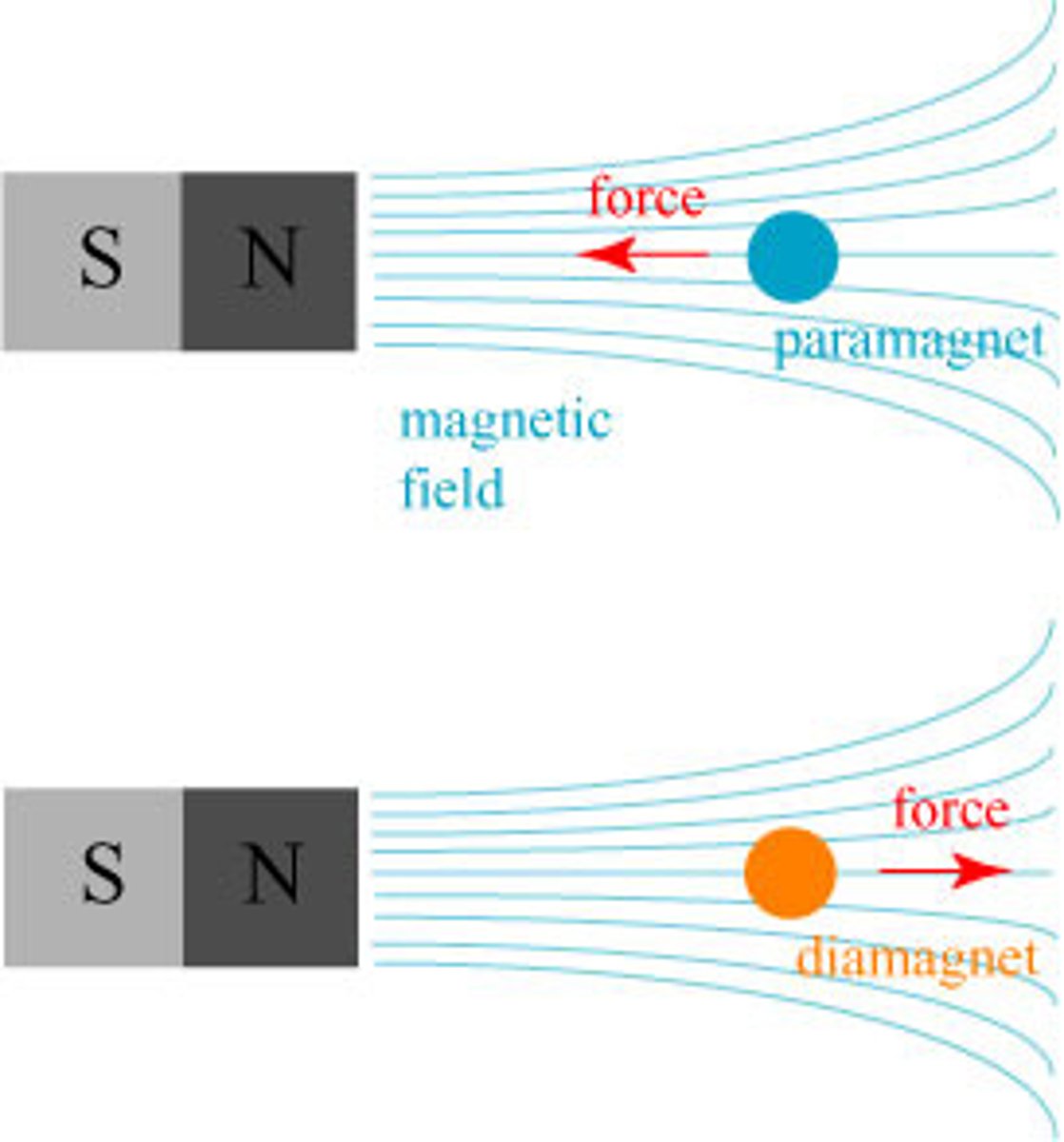
Paramagnetic Materials
- Materials have Unpaired electrons that align with magnetic fields, attracting material to a magnet.
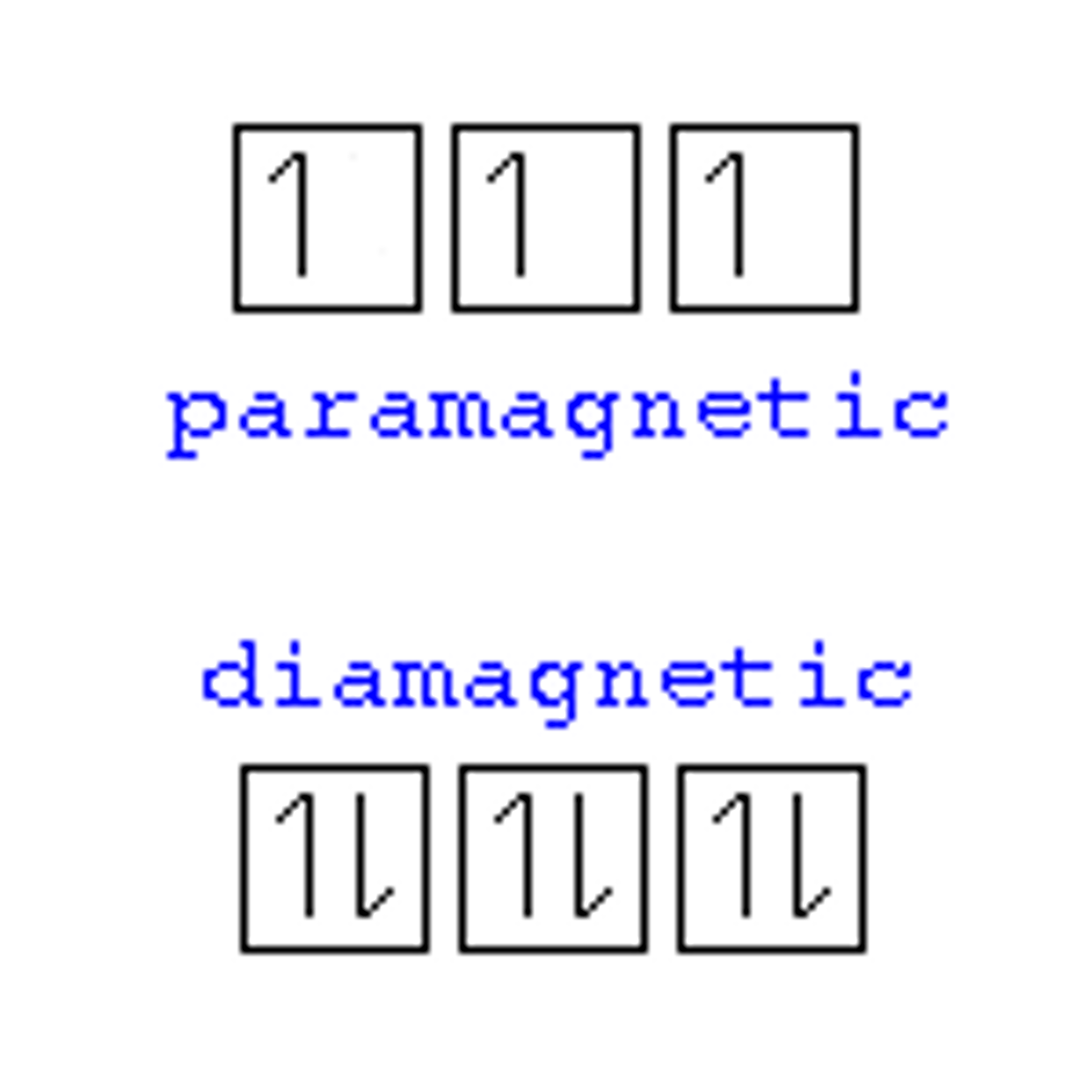
Diamagetic Materials
- Materials have all paired electrons, which cannot easily be realigned; they are repelled by magnets.
Maximum number of electrons within a shell
2n^2
Maximum number of electrons within a subshell
- 4l+2
- s = 2; p = 6; d = 10 ; f = 14
Effective Nuclear Charge (Zeff)
- Electrostatic attraction between the valence shell electrons and the nucleus.
- a measure of the net positive charge experienced by the outermost electrons.
- Zeff increases from left to right
- The values of q1 and q2 can represent the net charge of the nucleus and valence electron shell, respectively.
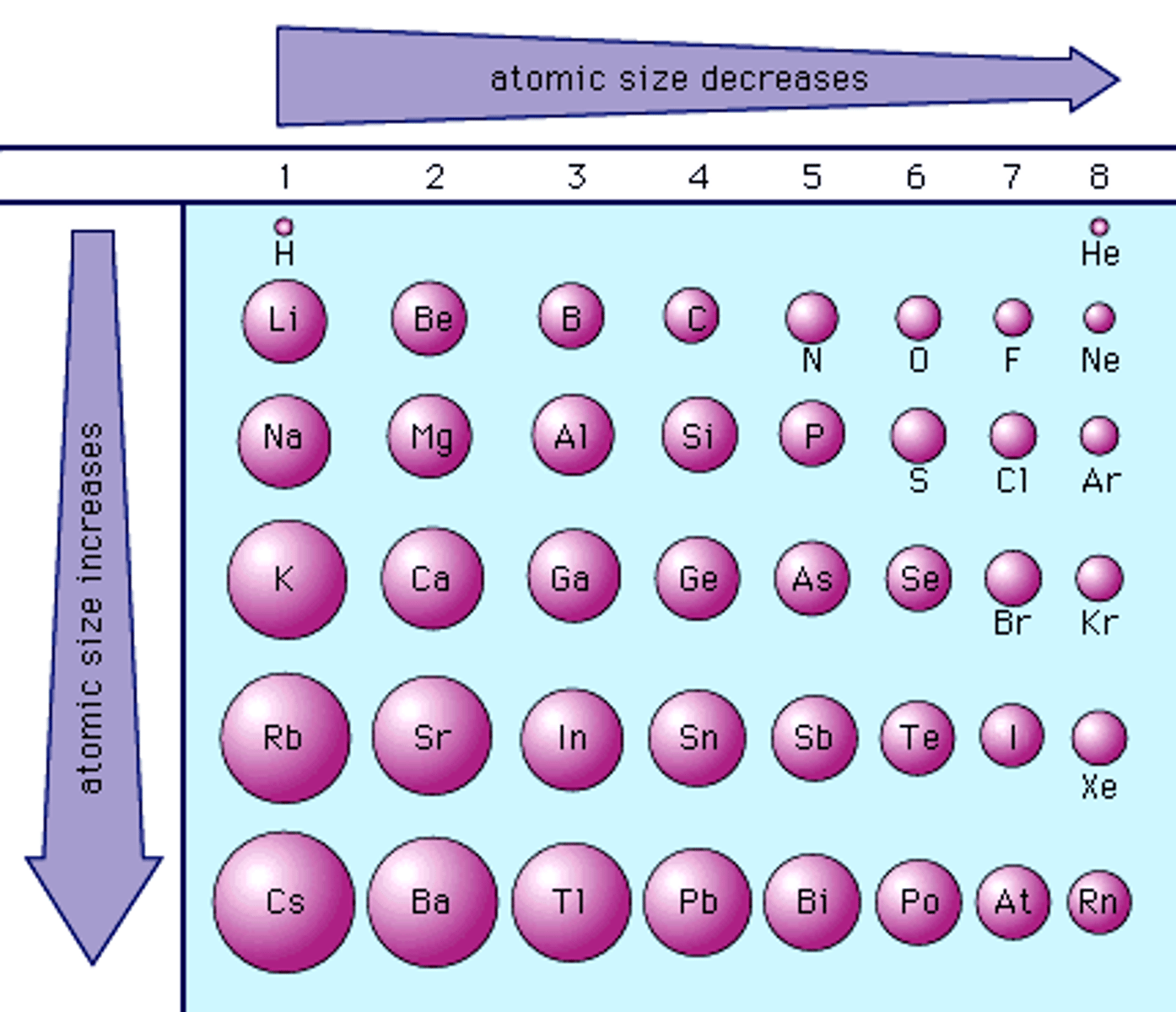
Atomic Radius
- Refers to the size of a neutral element.
- Zeff increases left to right across a period, as a result atomic radius decreases from left to right across a period.
- Increases down a group.
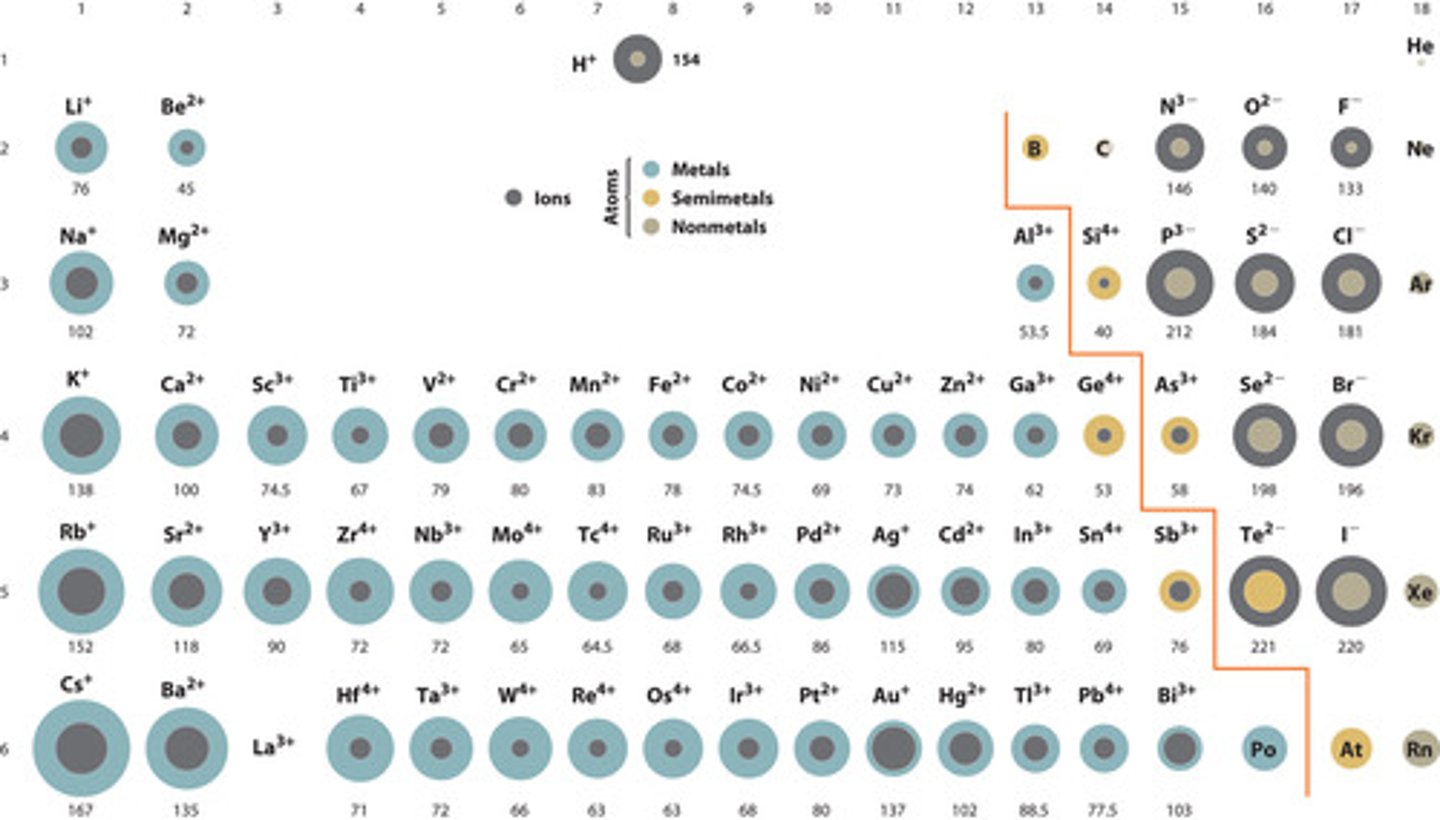
Ionic Radii
- Size of a charged species
- The largest nonmetal ionic radii and the smallest metallic ionic radii exist at the metalloid boundary.
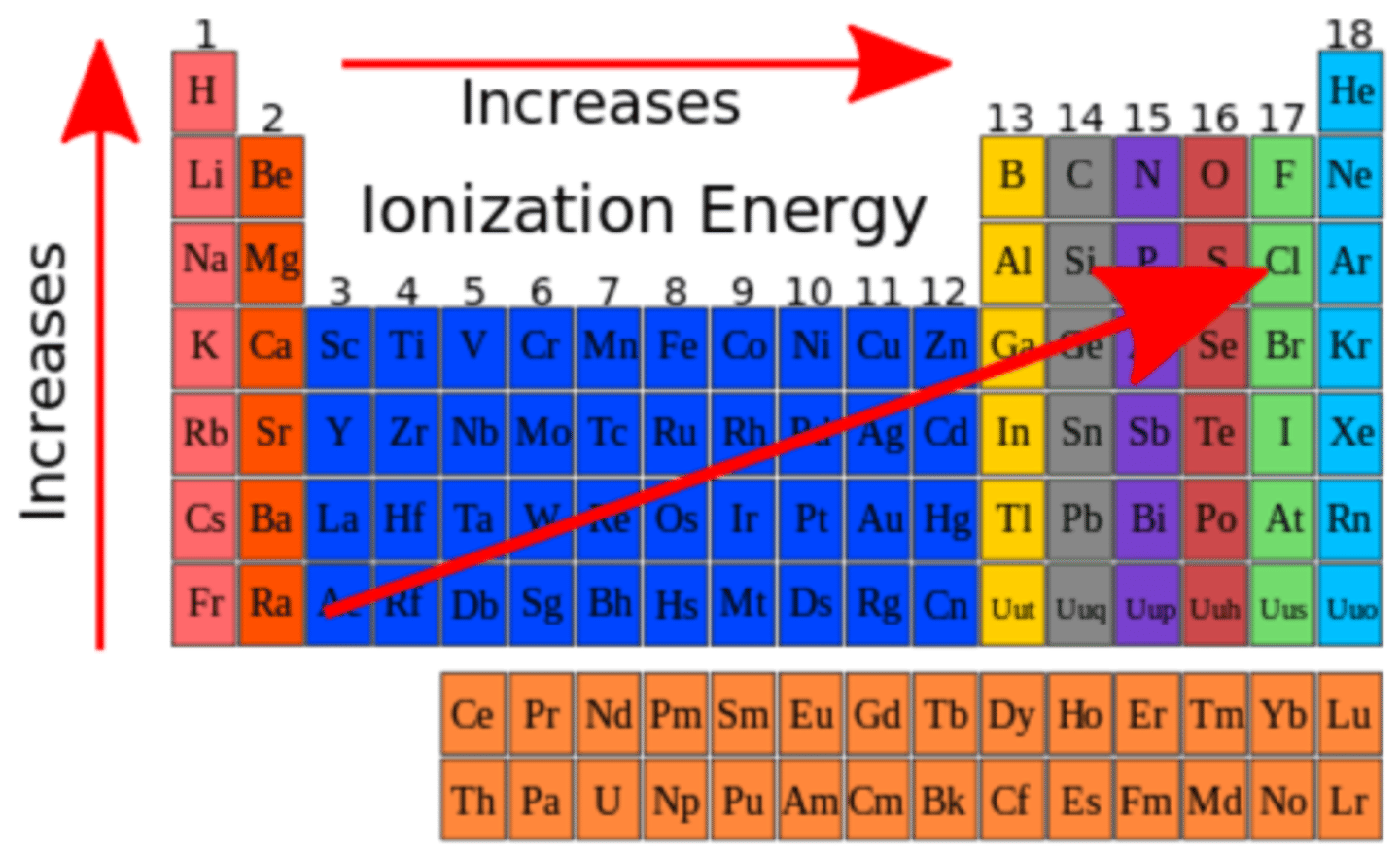
Ionization Energy
- The amount of energy necessary to remove an electron from the valence shell of a gaseous species.
- It is lowest when removal of the electron results in a complete shell or subshell; highest when the removal of the electron causes disrupts a complete shell.
- Increases from left to right a cross a period.
- 1st ionization energy < 2nd < 3rd
- Highest ionization energy = Most exothermic
- Ionization constant for weak acids is less than 1 (ex; 1.8*10^-5; only a very small amount dissociates in water)
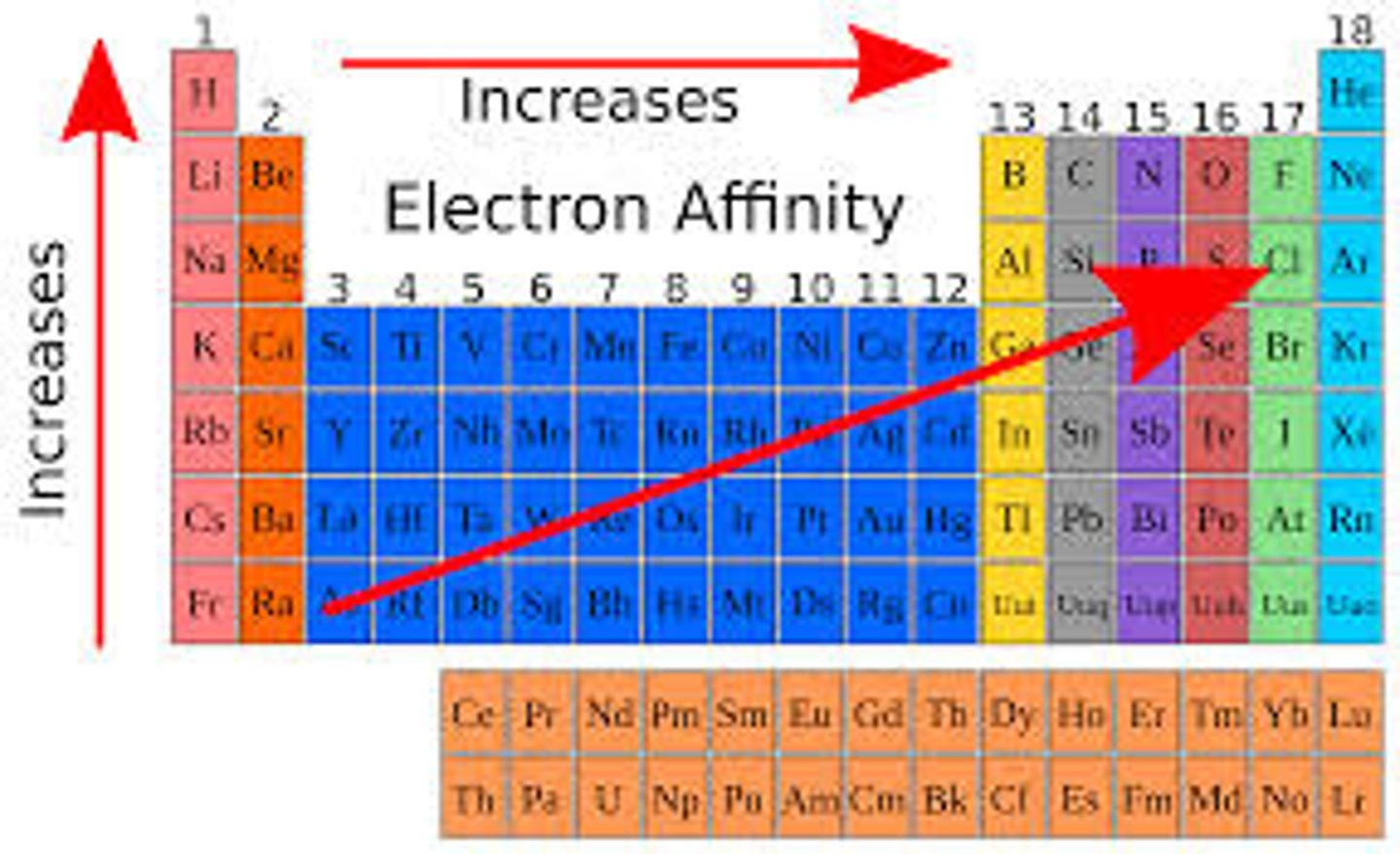
Electron Affinity
- Energy dissipated by a gaseous species when it gains an electron.
- If a reaction is exothermic then the electron affinity is positive.
Electronegativity
- A measure of the attractive force that an atom will exert on an electron in a chemical bond.
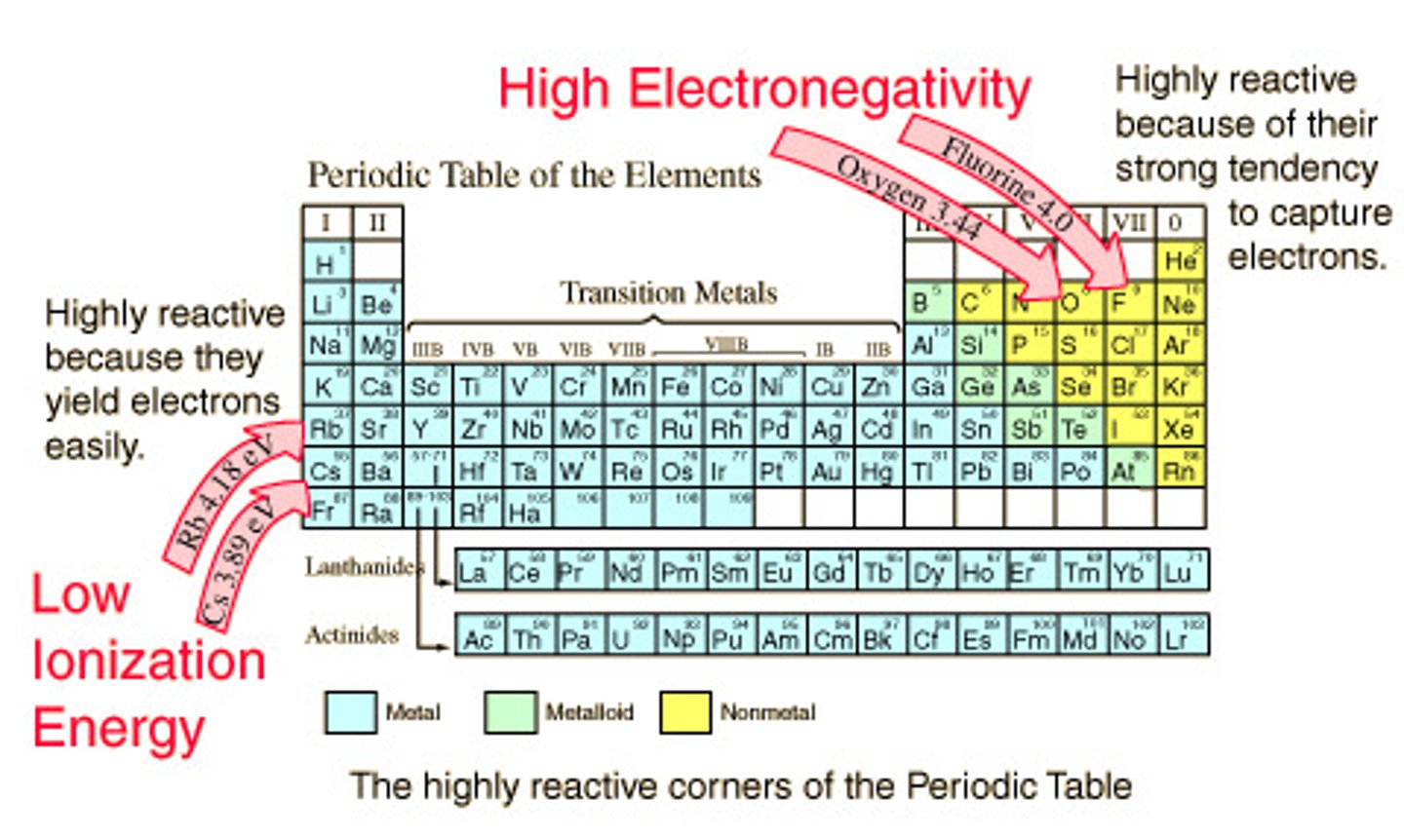
Alkali Metals
- Group 1A; active metals because they are so reactive and are not naturally found in their neutral state.
- Densities are lower than other metals
- Zeff values are very low which means low ionization energy, low electron affinity, and low electronegativity
- React violently with water; therefore, forming strong bases.
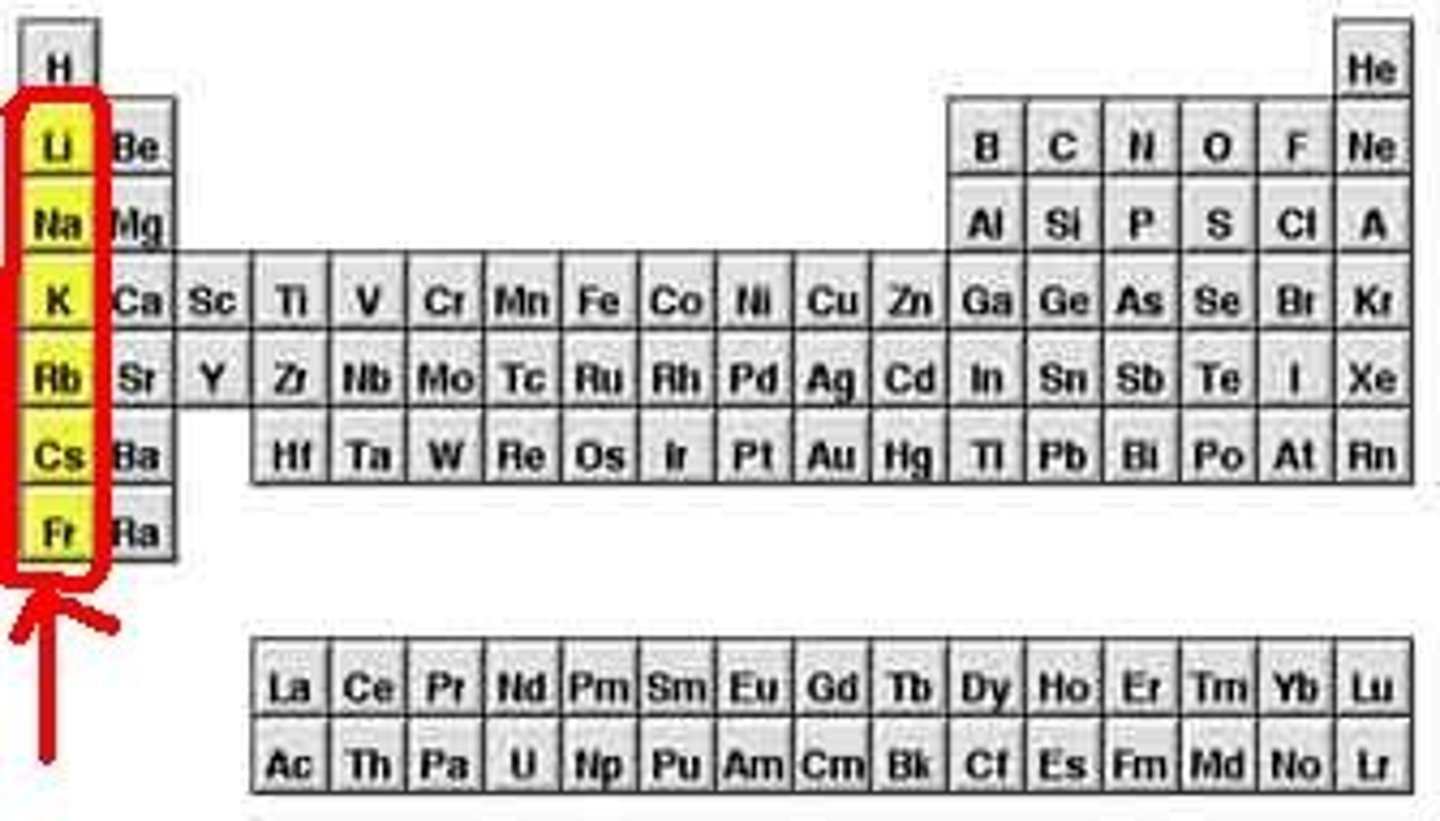
Alkaline Earth Metals
- Group 2; active metals because they are so reactive and are not naturally found in their neutral state.
- slightly higher nuclear charges, but though smaller atomic radii.
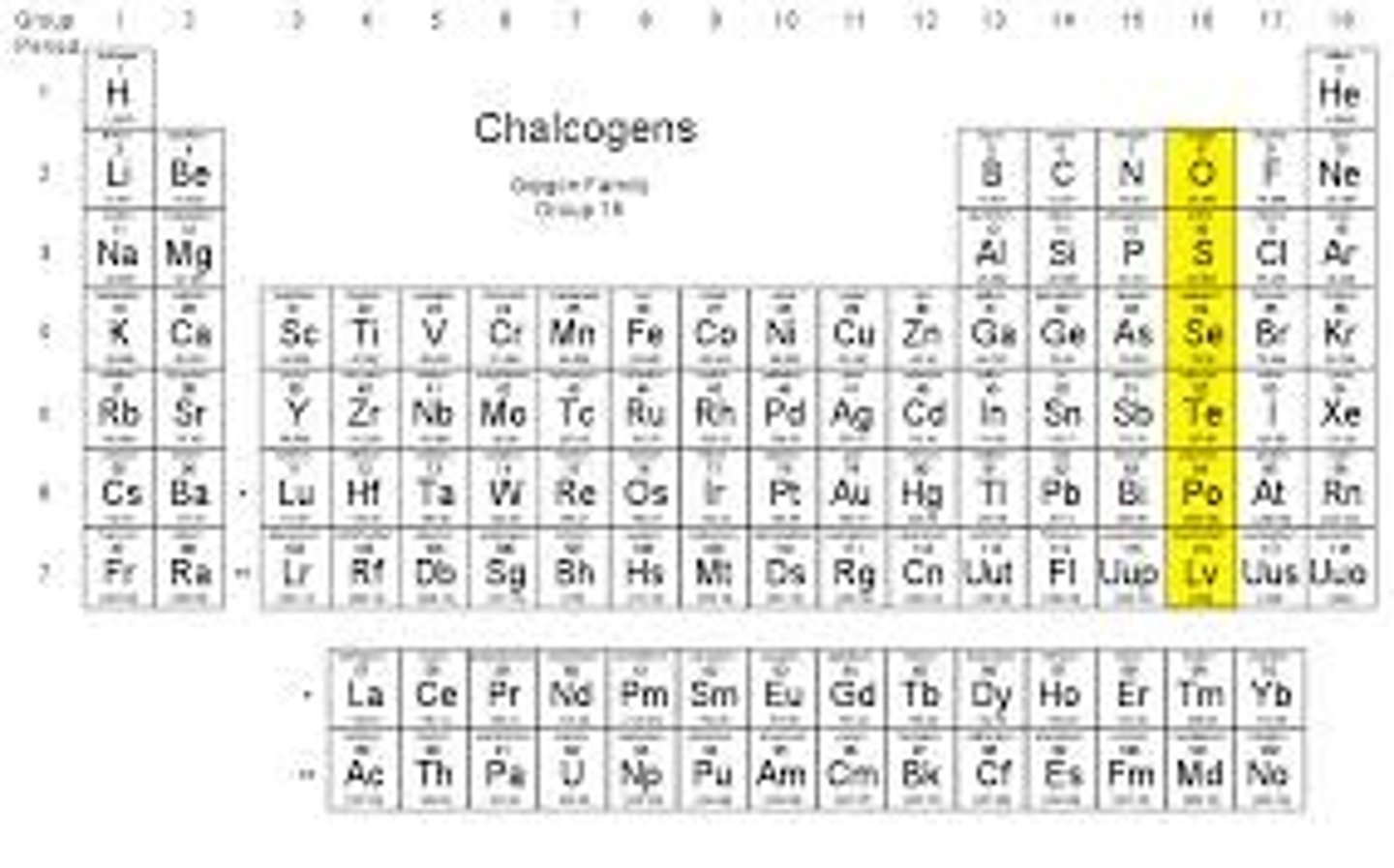
Chalcogens
- Group VIA or 16; Oxygen and sulfur
- Heavier ones are toxic but lighter ones are non toxic.
- small atomic radii & large ionic radii
Halogens
- Group VIIA or Group 17
- Highly reactive non metals; desperate to complete their valence electrons.
- Mostly found in diatomic molecules.
- Gaseous ( F2 & Cl2); Solid (Br2); Liquid (I2)
Nobel Gases
- Minimal chemical reactivity
- High Ionization energy
- Extremely low boiling point & exist as gases at room temperature.
- Virtually nonexistent electron negativities and electron affinities.
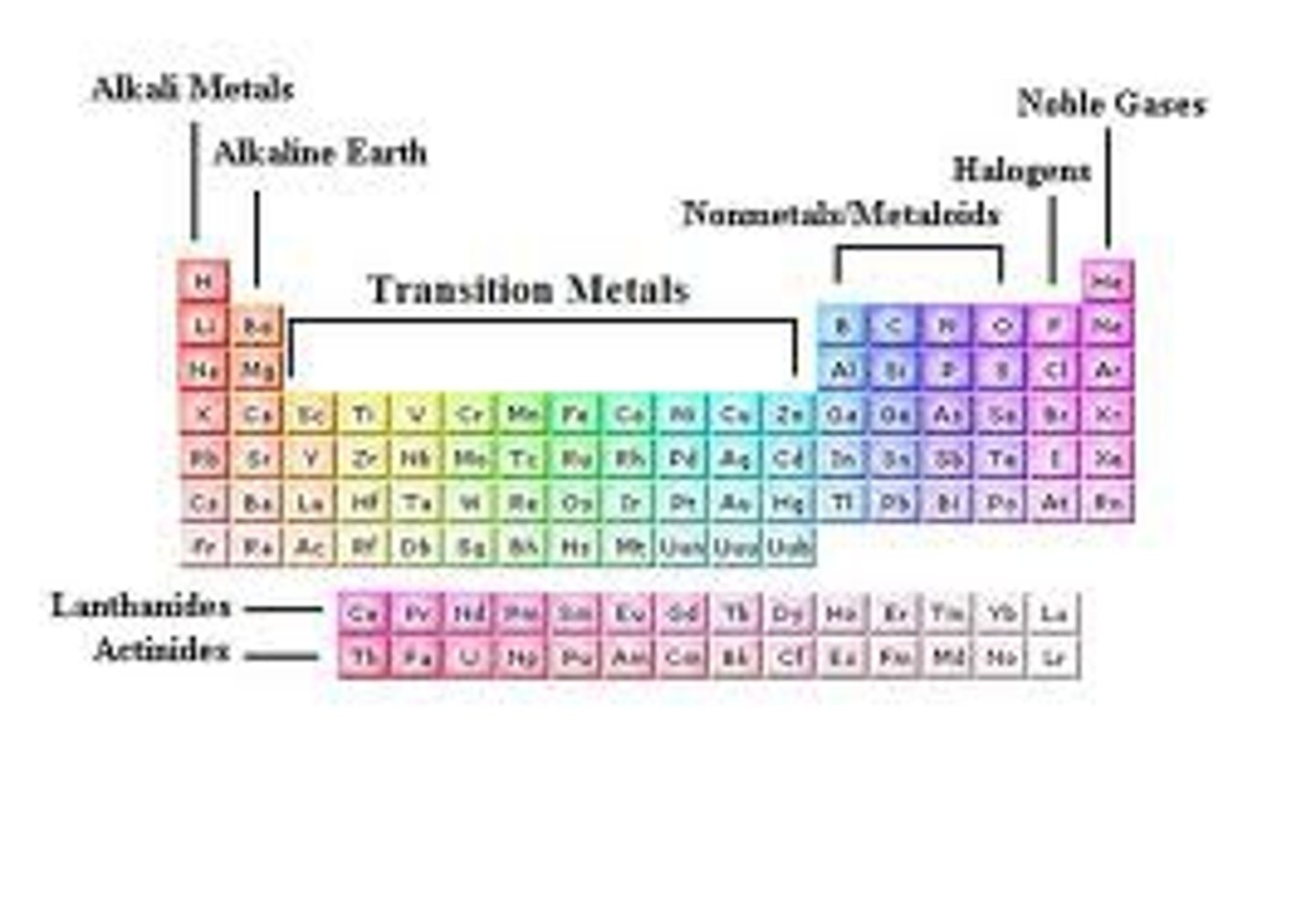
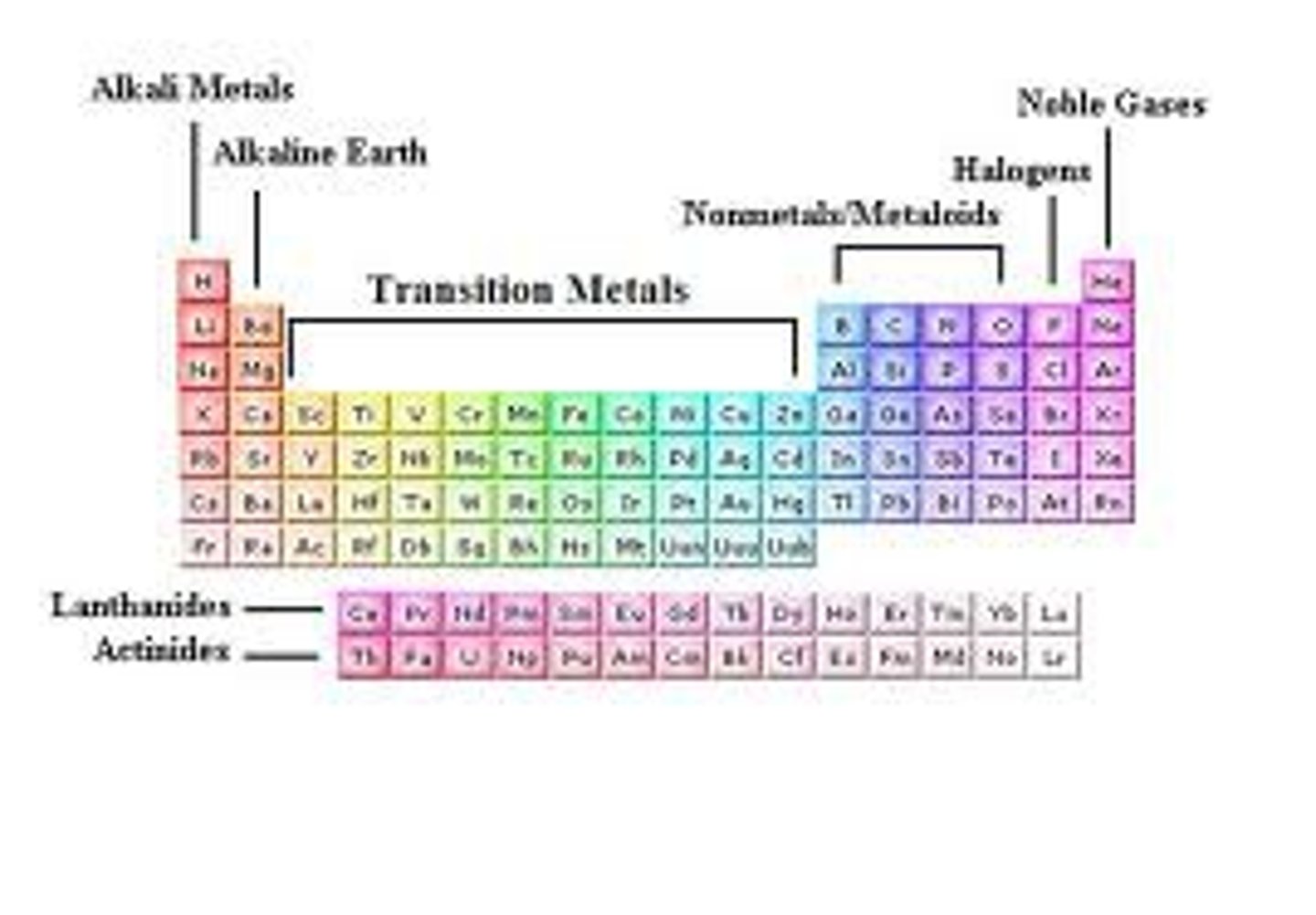
Transitional Metals
- They take on multiple oxidation states, which explains their ability to form colorful complexes with nonmetals in solution and their utility in certain biological systems.
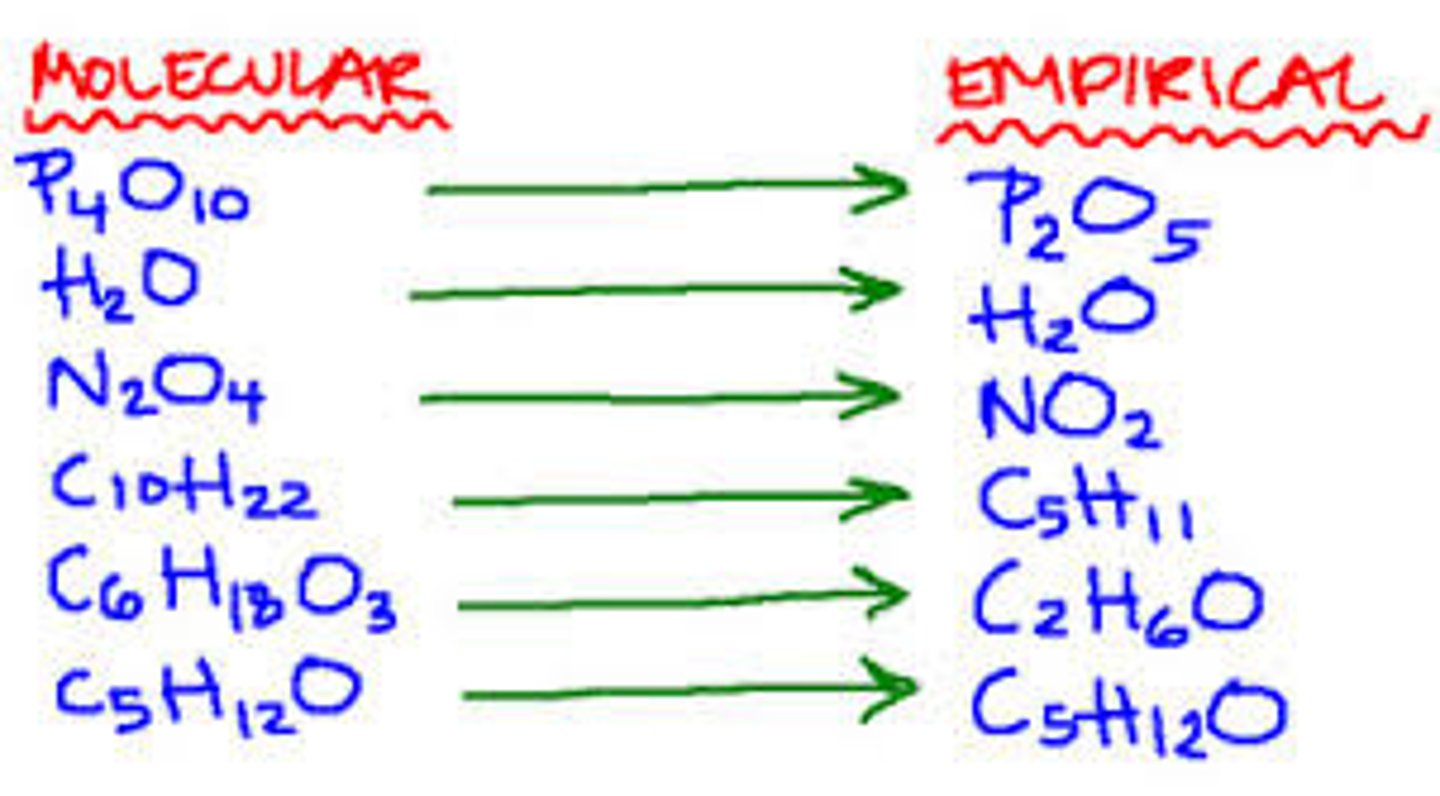
Empirical Formula
- Gives the simplest whole number ratio of the elements in the compound.
- CH_2O indicative of monosaccharide include glucose, fructose, and galactose.
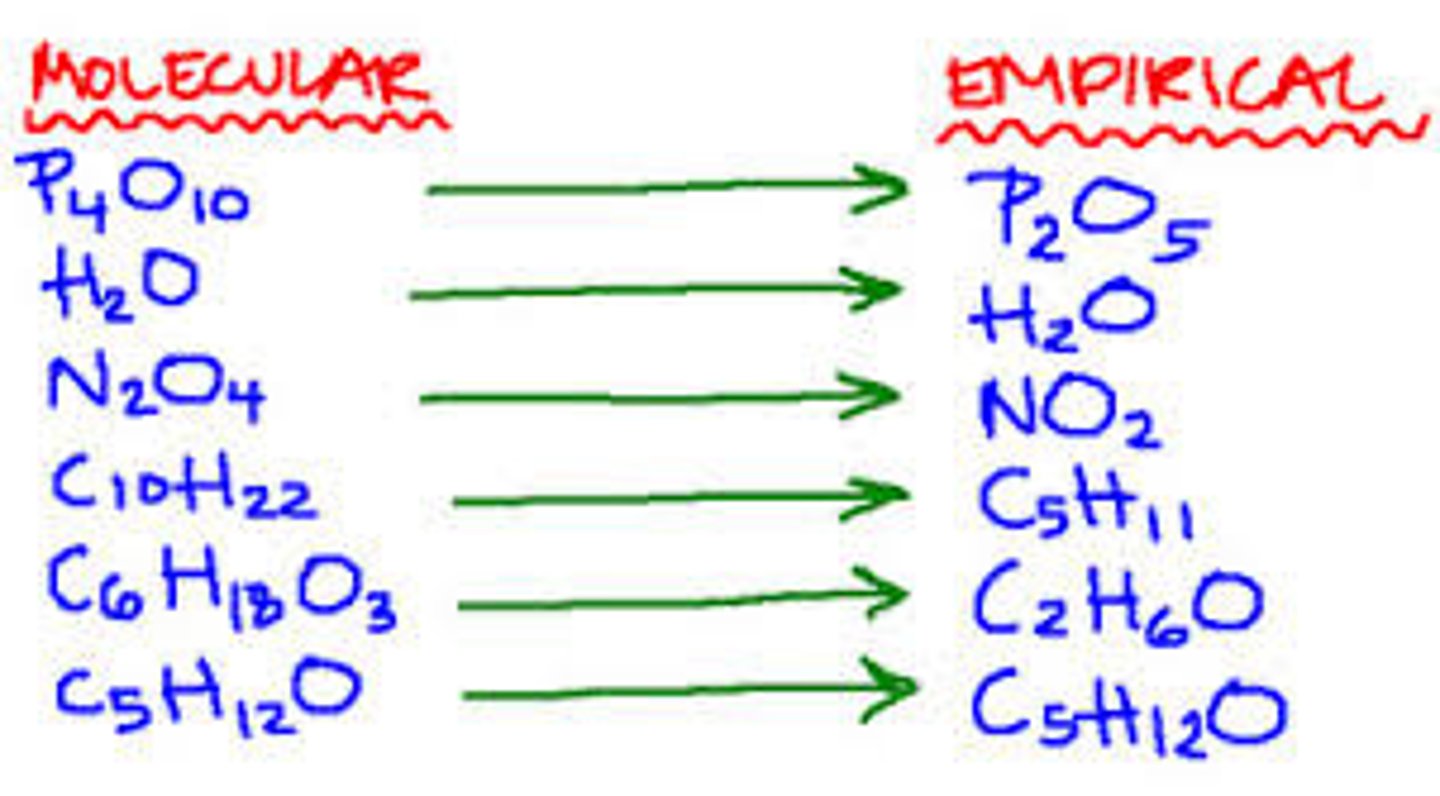
Molecular Formula
Gives the exact number of atoms of each element in the compound and gives the exact number of atoms of each element in the compound.
Combustion Reaction
- A special type of reaction that involves a fuel, usually a hydrocarbon and an oxidant ( normally oxygen).
- Products are usually water and carbon dioxide
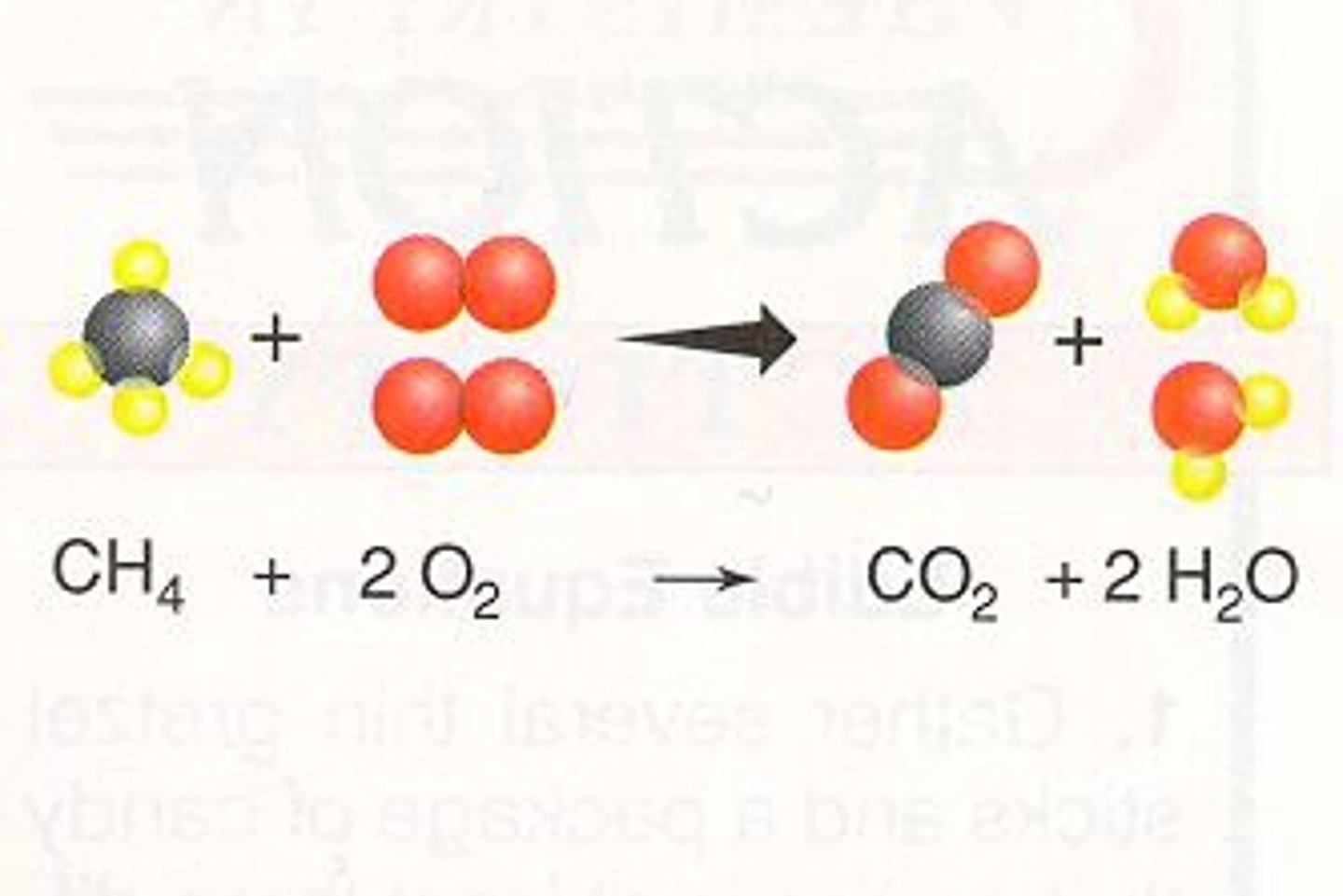
Electrolyte
- Solutes that enable solutions to carry currents
- Ionic compounds such as NaCl, and KI.
- Highly polar covalent bonds
- Dissociate into ions when dissolved
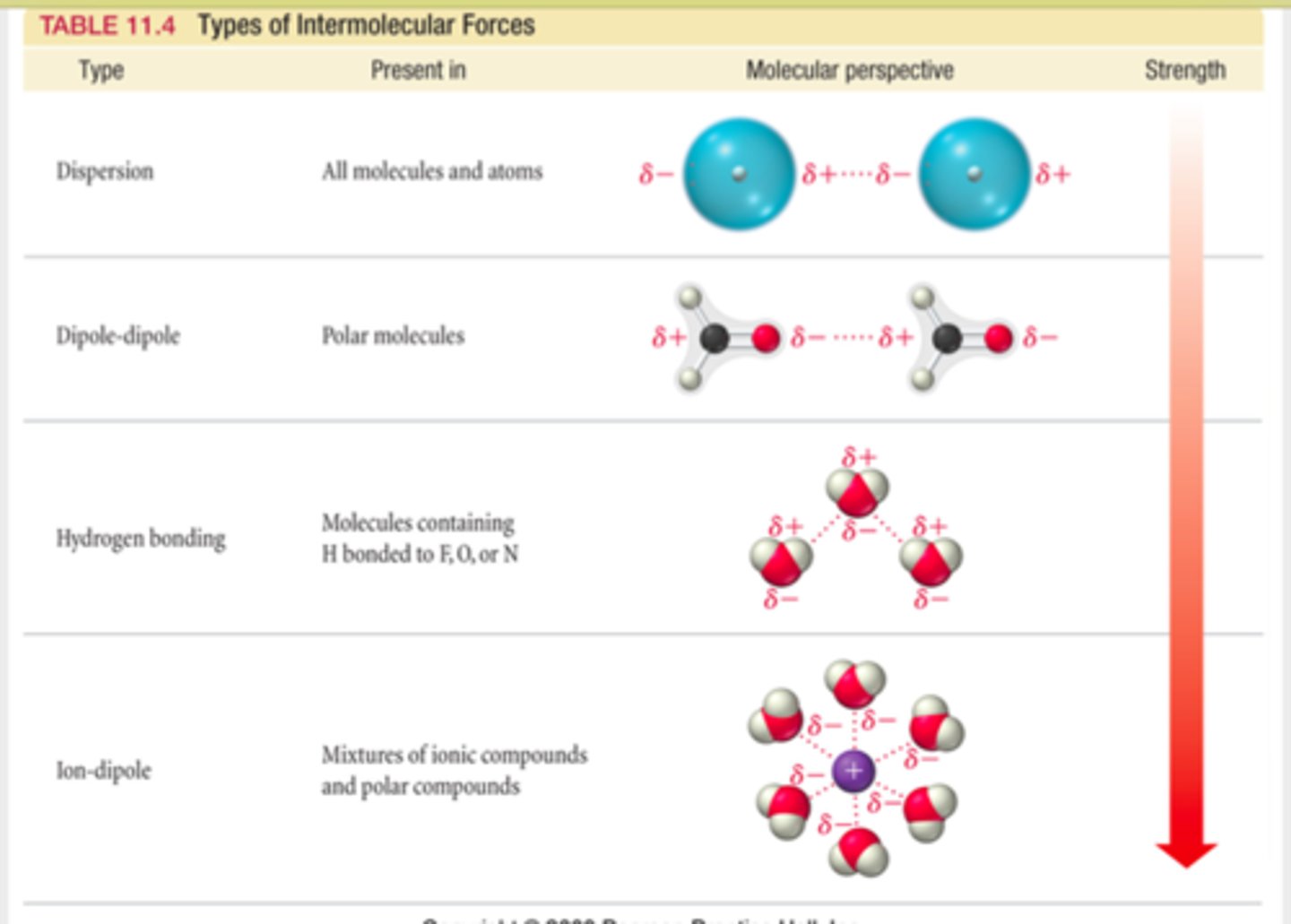
Intermolecular Forces
- Larger intermolecular forces correspond to higher boiling points.
- London Dispersion: nobel gas with a full octet; it's why they can liquefy.
- Dipole- Dipole interactions: polar molecules such as Acetone and Isopropyl alcohol ( it can also form hydrogen bonds)
- Ionic Bonds : such as KCl
Carbonate
- Trigonal Planar: no lone pair
- Double bond with one of the oxygens
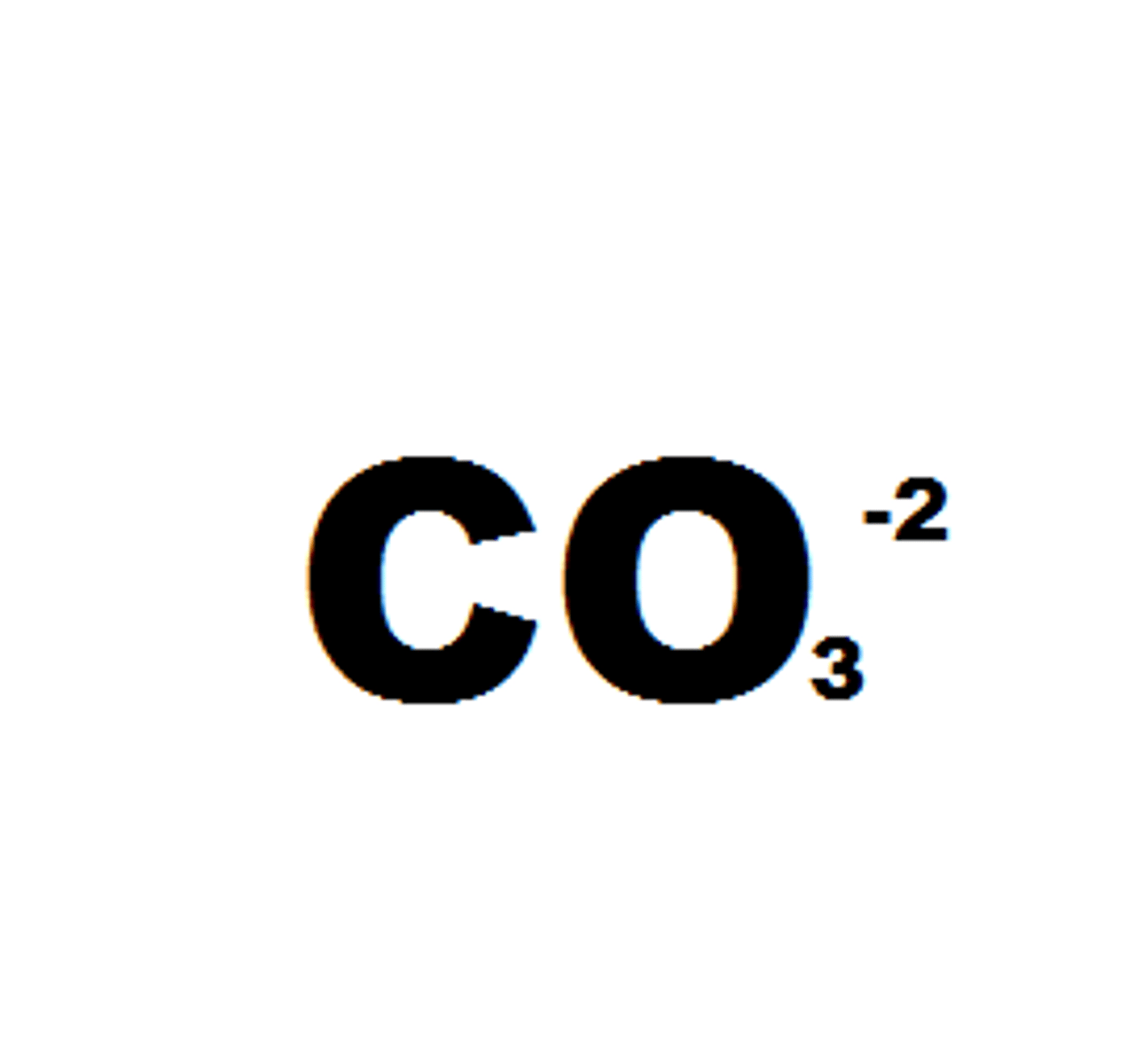
Trigonal Pyramidal
- 2 lone pairs
- ClF3
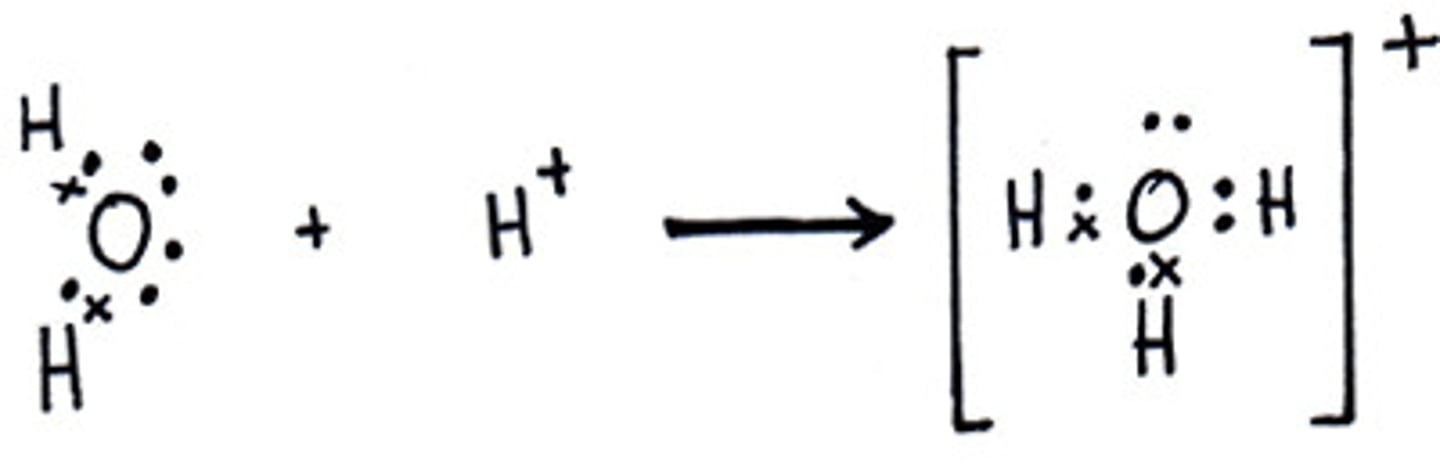
Coordinated covalent bond
- This represents the donation of a shared pair of electrons from a lewis base (H2O) to a lewis Acid (H+, electron Acceptor)
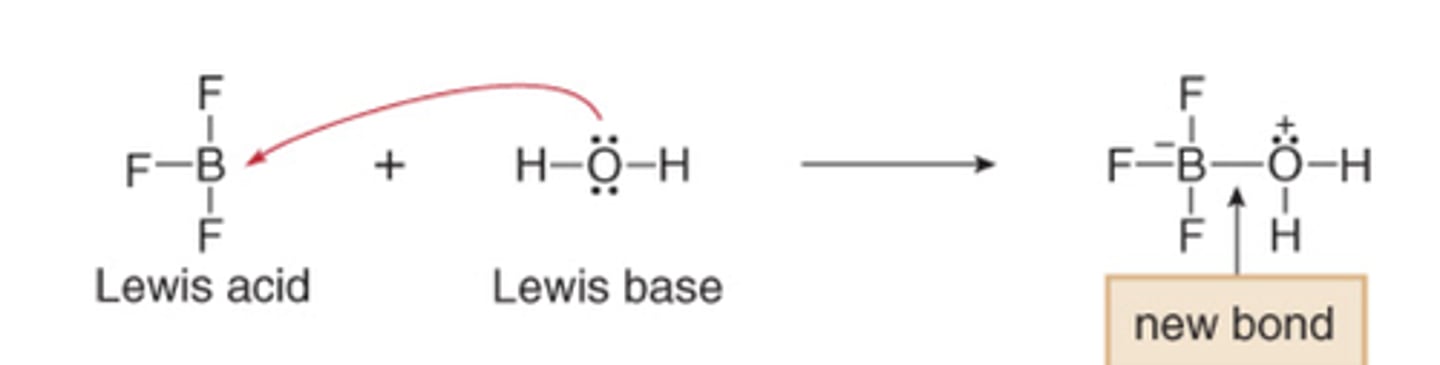
Lewis Acid
- Electron Acceptor
- Electrophile
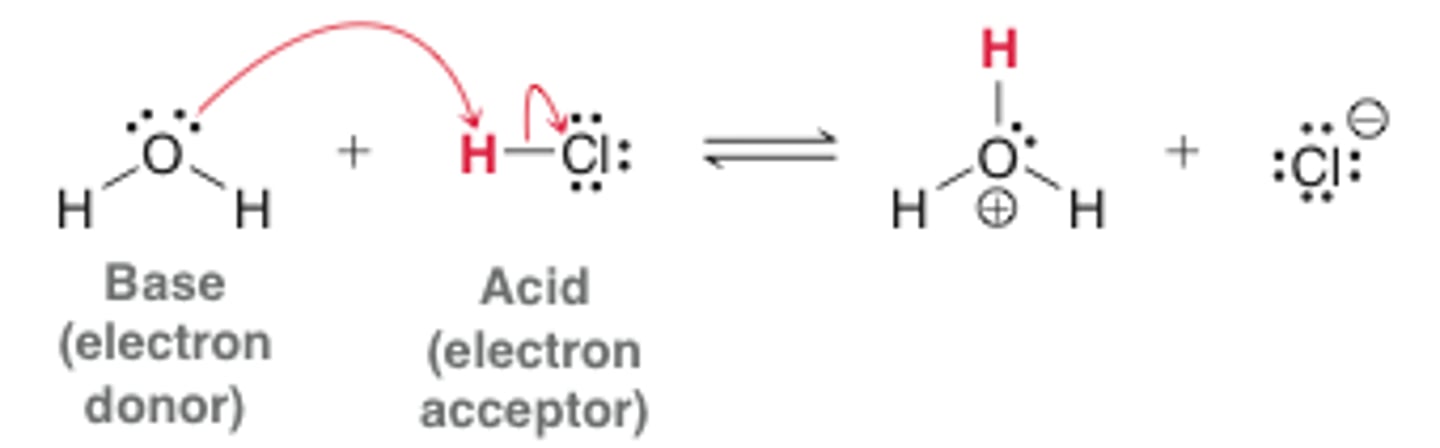
Lewis Base
- Electron Donor
- Nucleophile
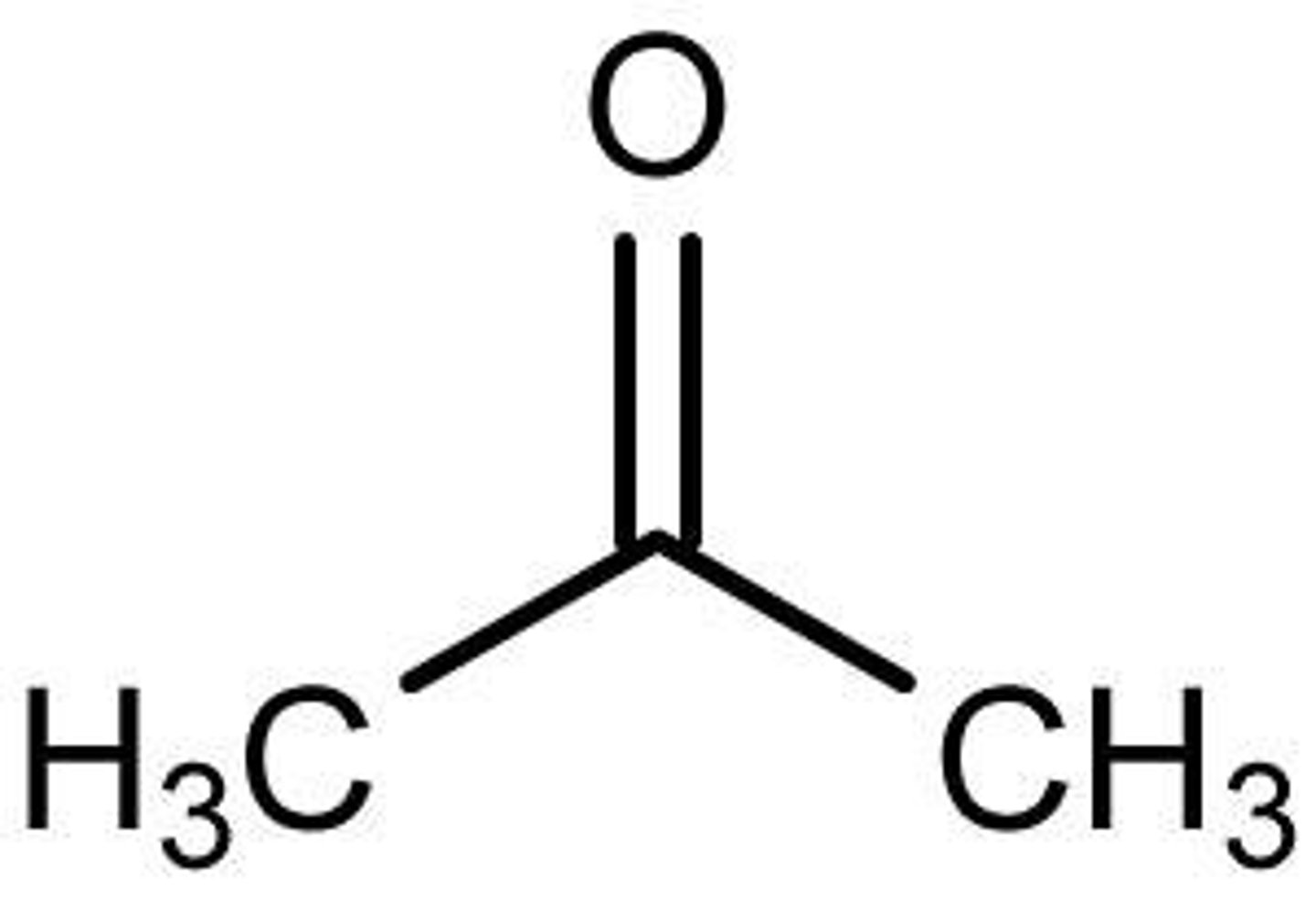
Acetone
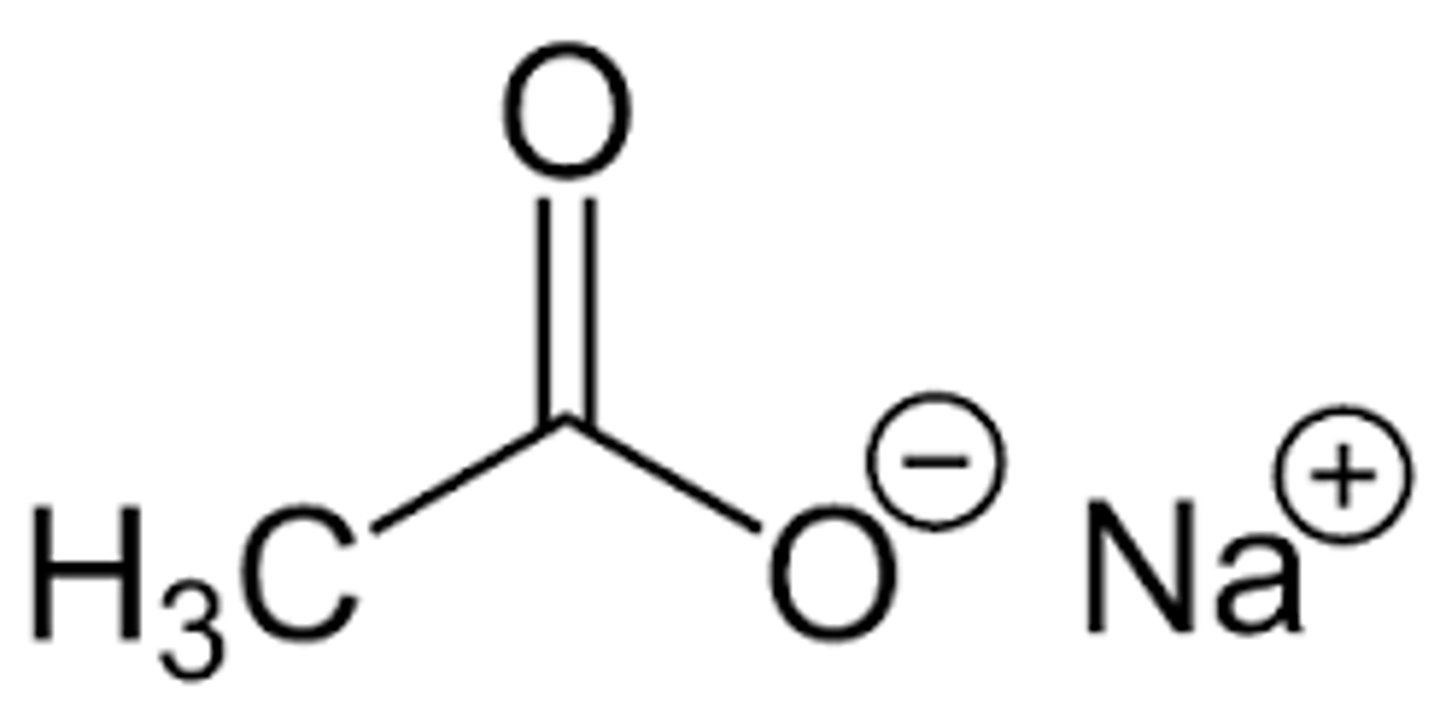
Sodium Acetate
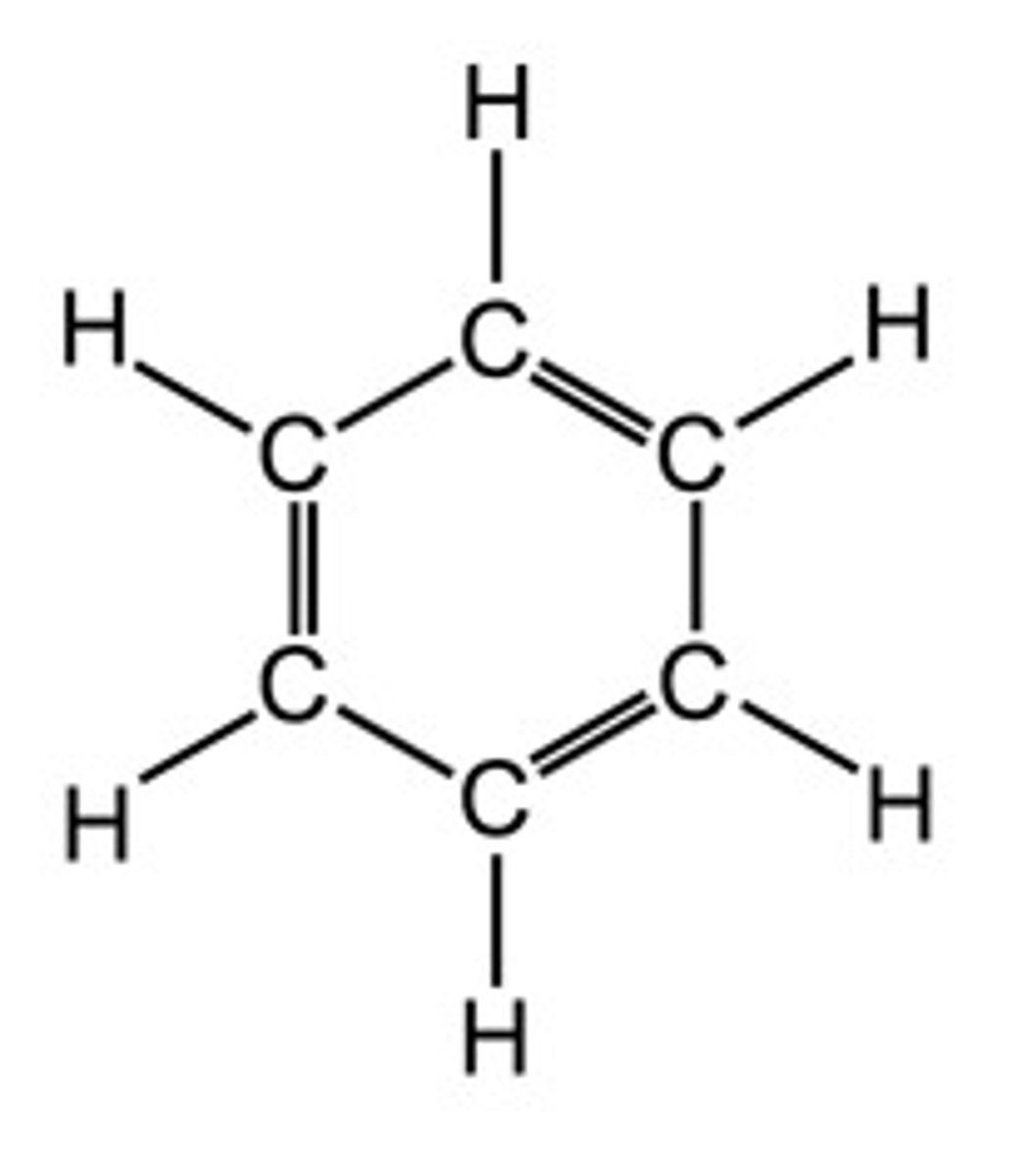
Benzene
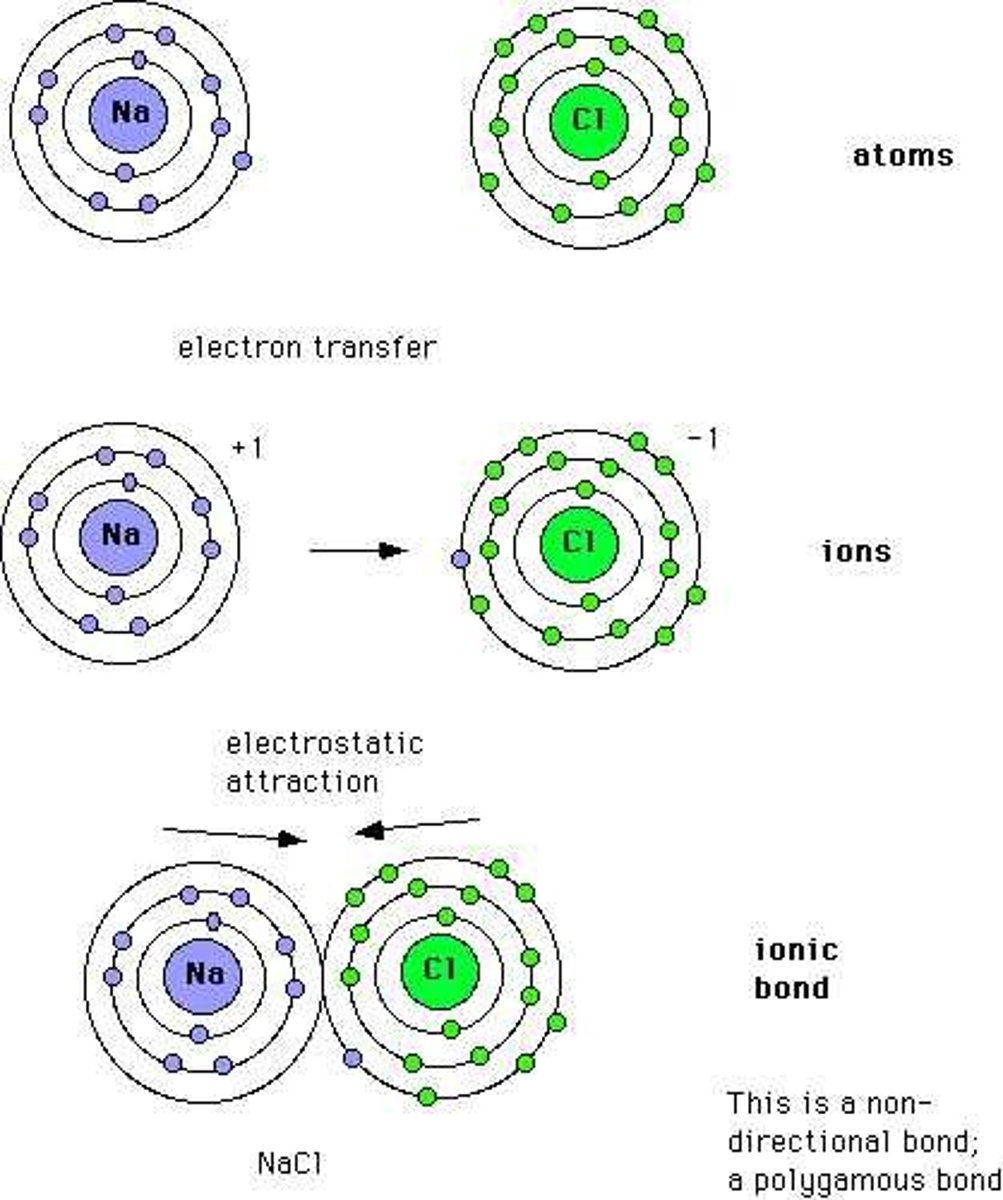
Ionic Compound
Three Dimensional arrays of charged particles.
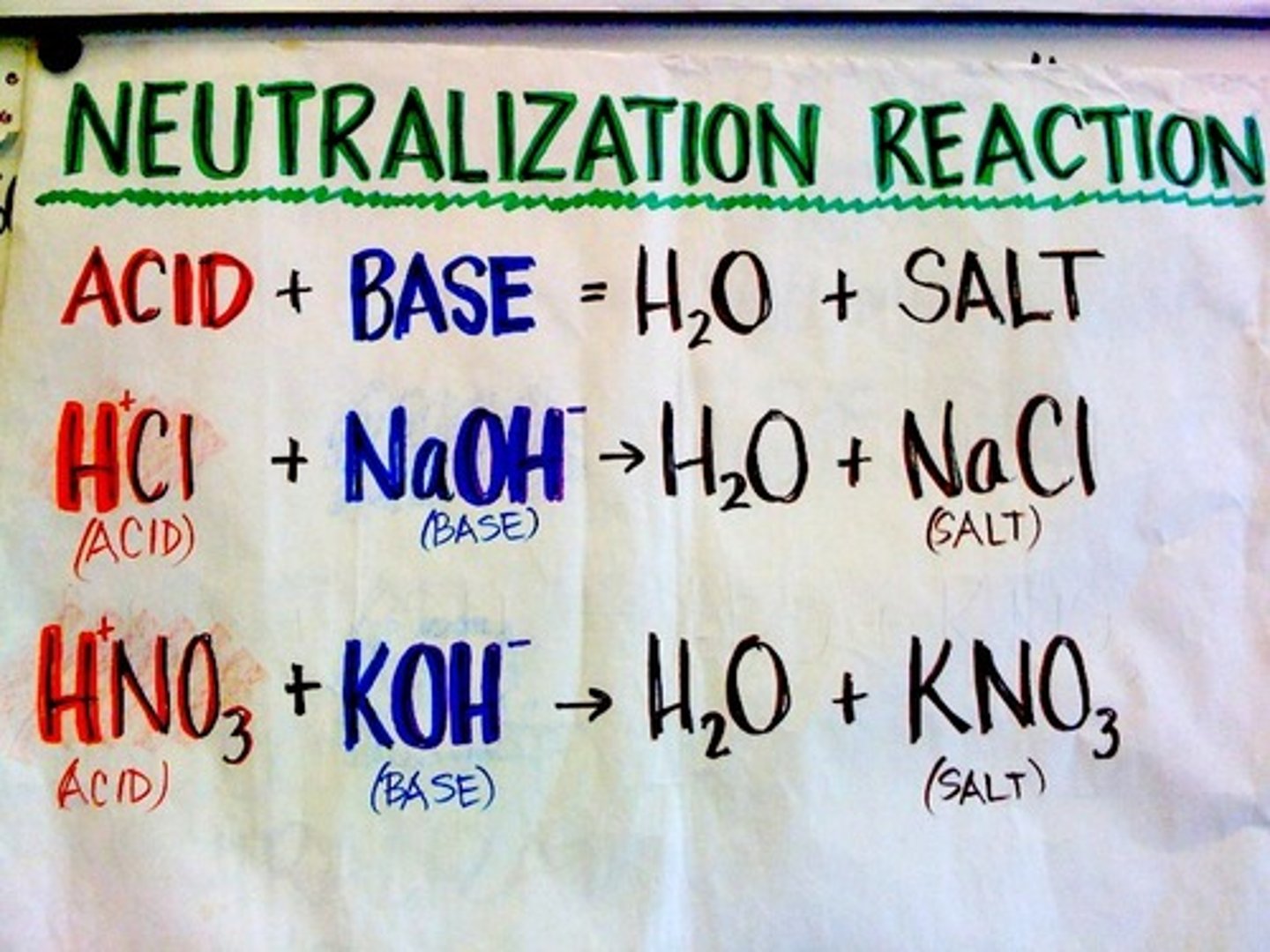
Neutralization Reaction
- Ca(OH)2 + H2SO4 -> CaSO4 +H2O
- Acid & base = Salt and water
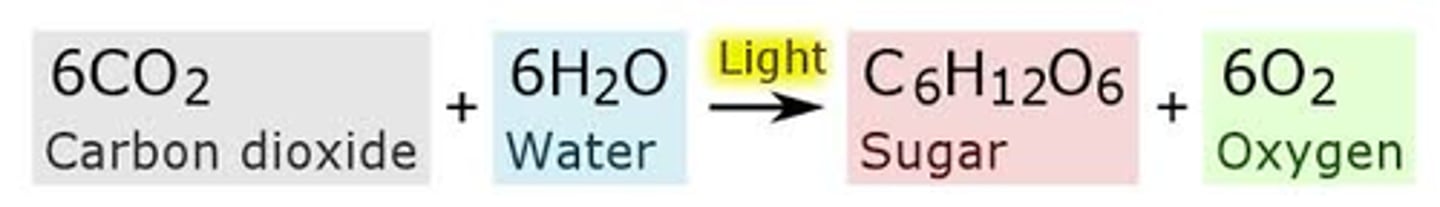
Photosynthesis Equation
Glucose: 180 g/mol
Water: 18 g/mol
CO2: 46 g/mol
Gibbs Free Energy
- Thermodynamic Potential
- Spontaneous: Negative; Exergonic; ex) utilization of ATP; Enthalpy must be negative and entropy must be positive
- Non-spontaneous: Positive; Endergonic
- Free energy of the product can be raised or lower but not the activation energy value.
- Delta G is temperature dependent when Delta H and Delta S have the same sign.
- Negative Delta H and Positive Delta S = always spontaneous.
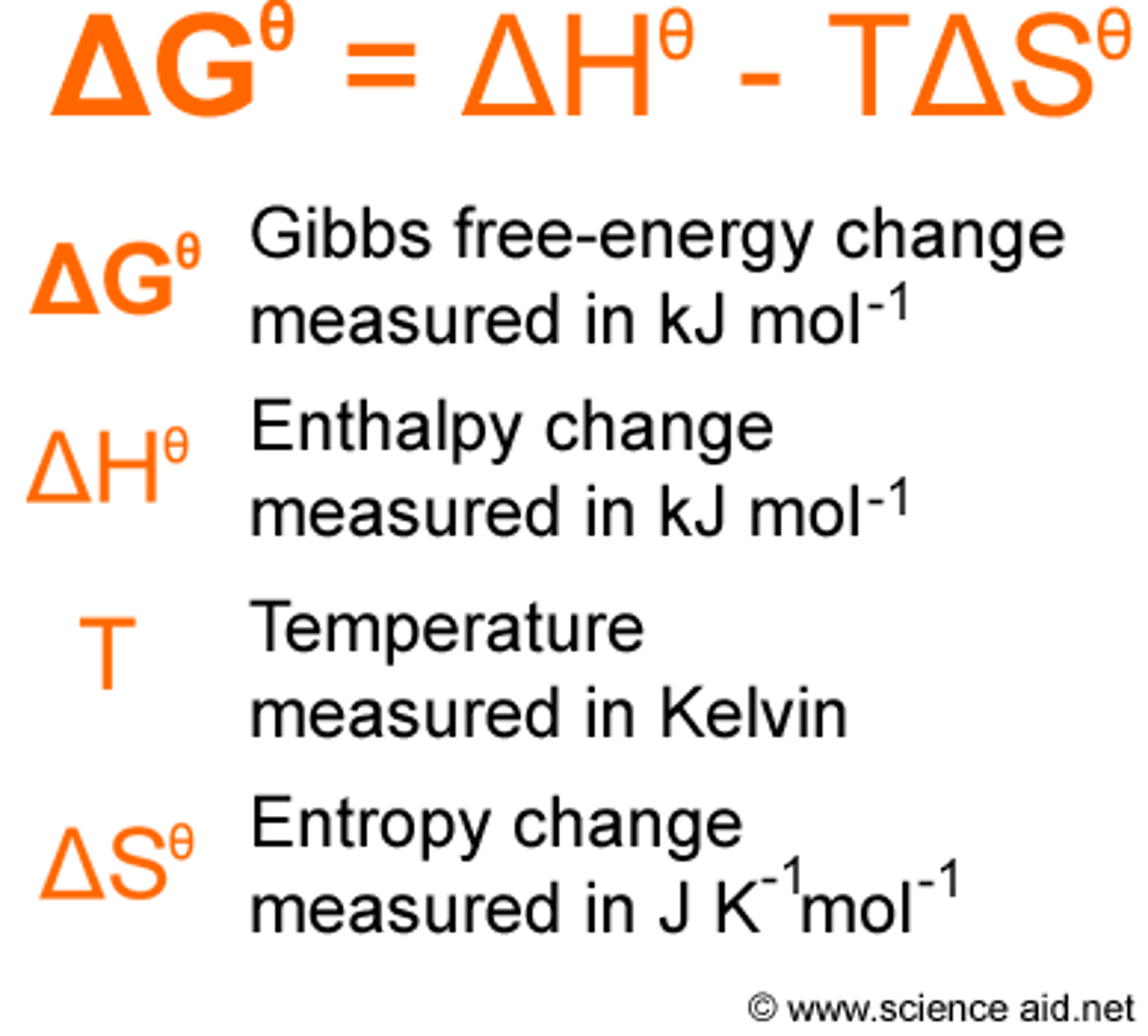
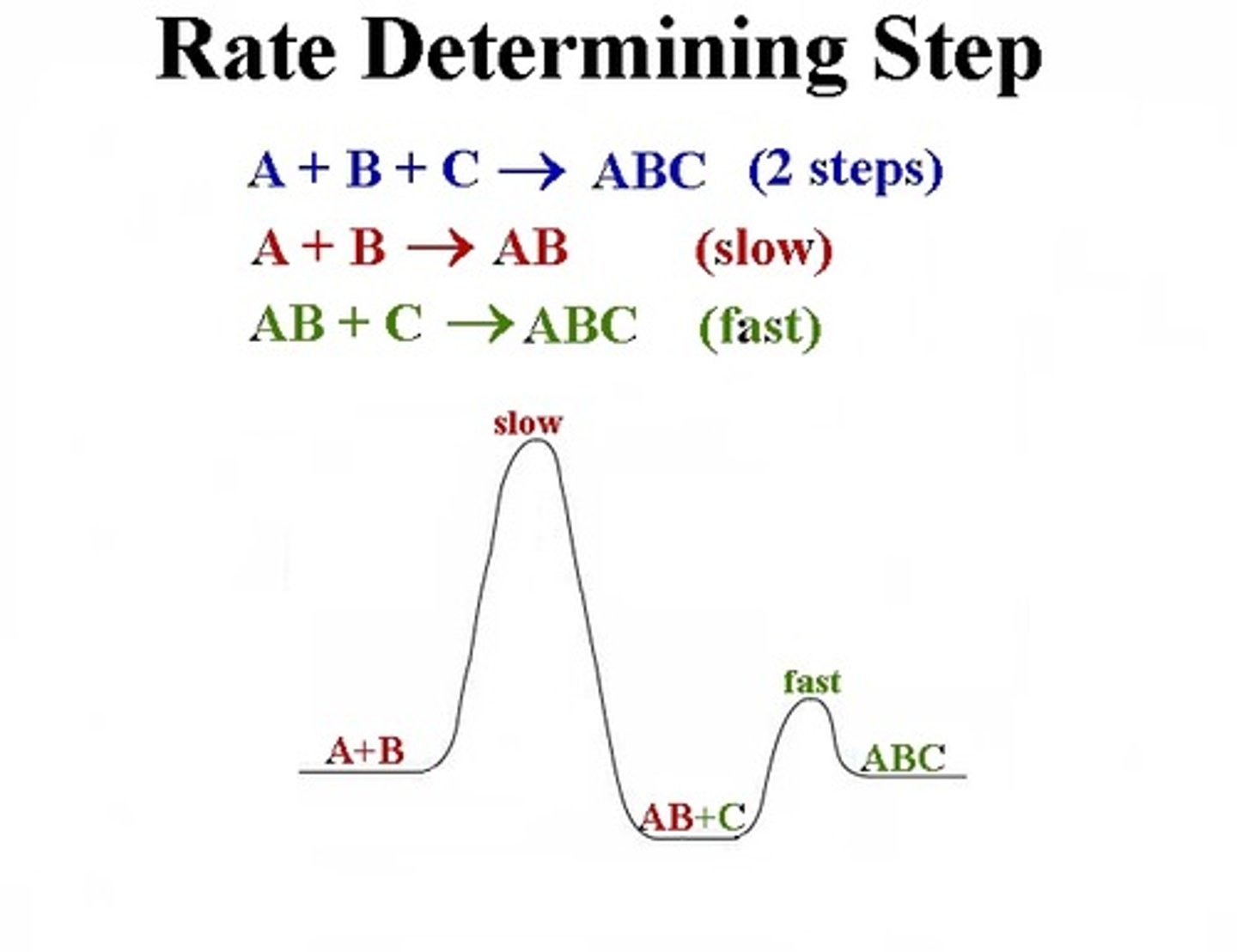
Rate Determining Step
- The rate of the whole reaction is only as fast as the rate determining step.
- Slowest step in any proposed mechanism
- AB is the intermediate
Collision Theory of Chemical Kinetics
- The rate of the reaction is proportional to the number of collisions per second between the reacting molecules.
- Not all collisions result in a Chemical reaction
- Effective collision = leads to product
- Greater the concentration of the reactants, greater the number of effective collision
- Z : total number of collisions; F: fraction of collisions that are effective
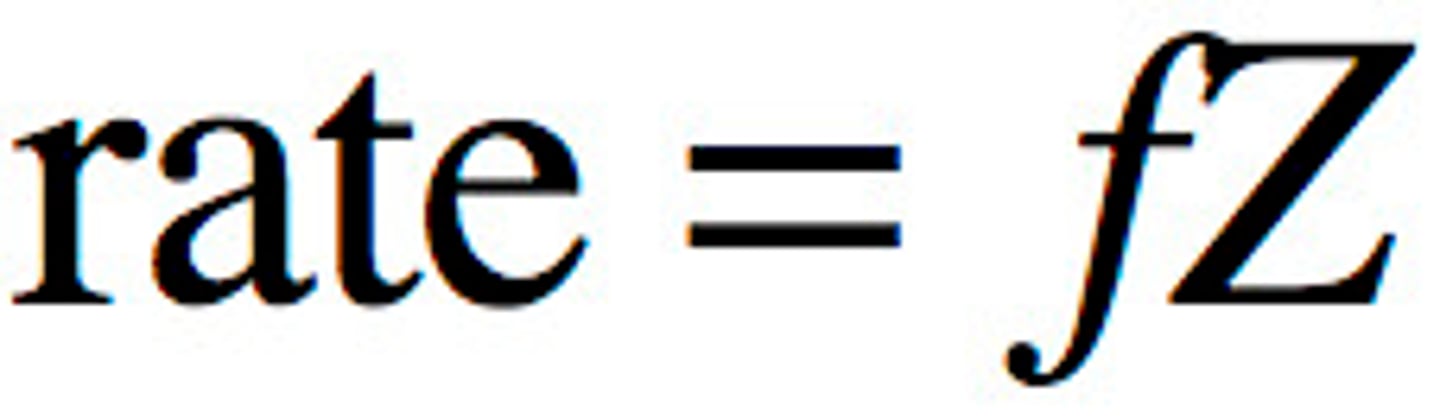
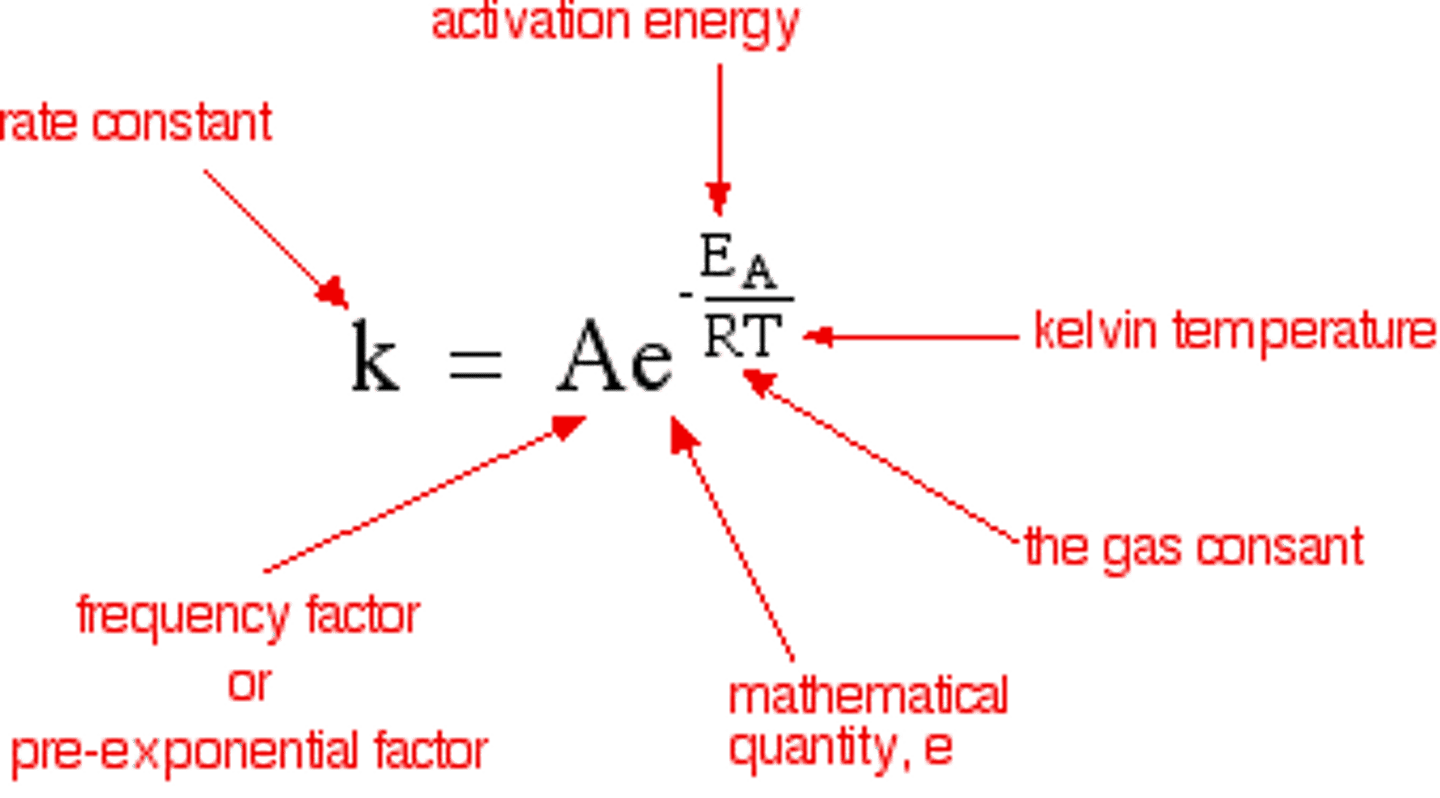
Arrhenius Equation
- R: gas constant value is 8.3144598(48) J mol−1 K−1
- If the temperature was to increase to infinity, then the exponent would have a magnitude less than 1.
- However; note the presence of a negative sign. As the magnitude of the exponent gets smaller, meaning more towards zero, rate constant actually increases.
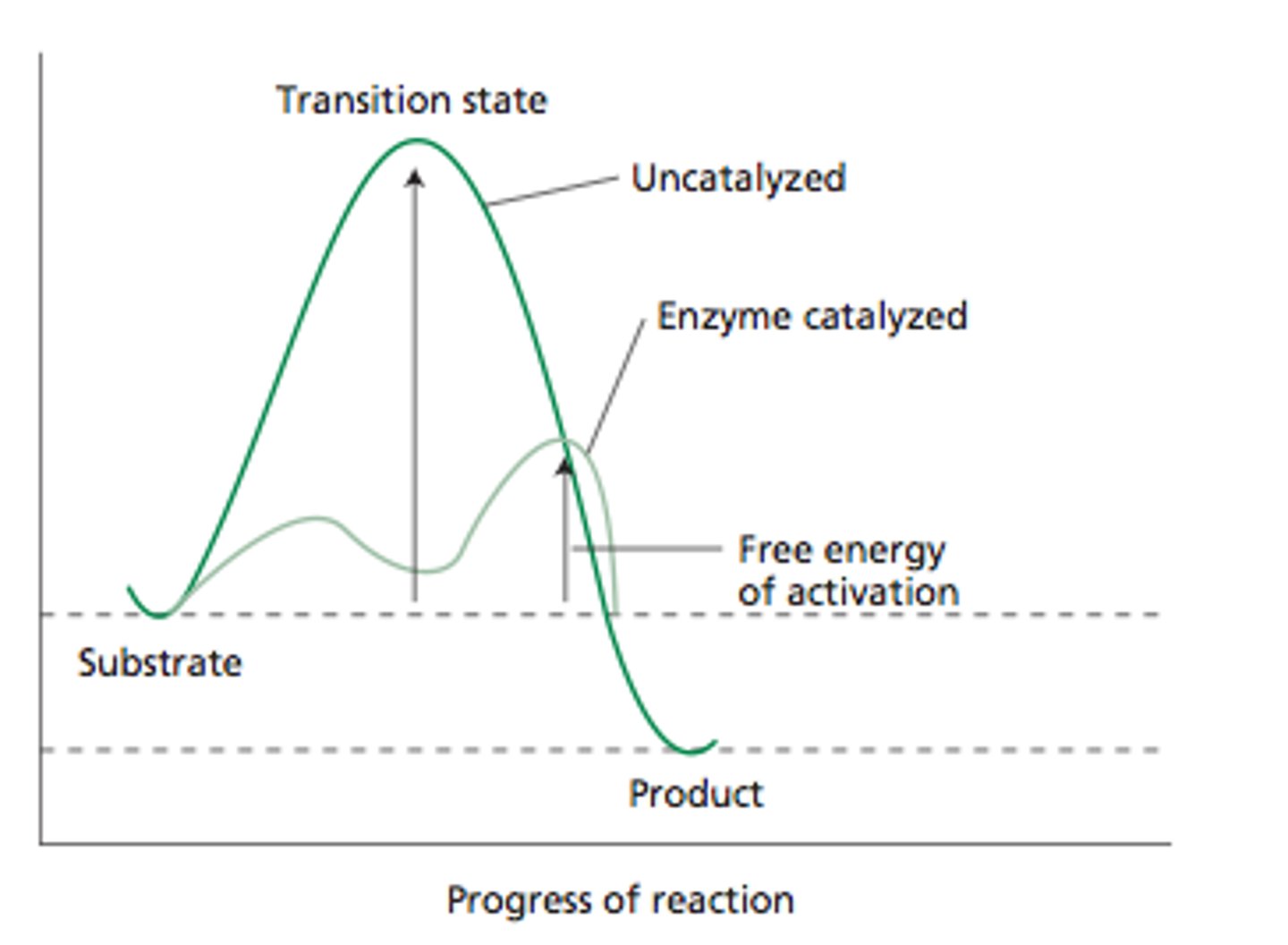
Transition State
- Molecules collide with energy equal to or greater than the activation energy
- Has greater energy than both the reactant and the product
Medium
- The rate of the reaction may also be affected by the medium in which it takes place.
- Generally, Polar solvents are preferred because their molecular dipole tends to polarize the bonds of the reactants, thereby lengthening and weakening them, permitting the reaction to occur faster.
Catalyst
- Inc. reaction rate without being consumed in the reaction.
- Dec. in the energies of the activation energy, for both forward and reverse rxn.
- no impact on equilibrium position, Keq..... they will not transform a non spontaneous reaction to spontaneous.
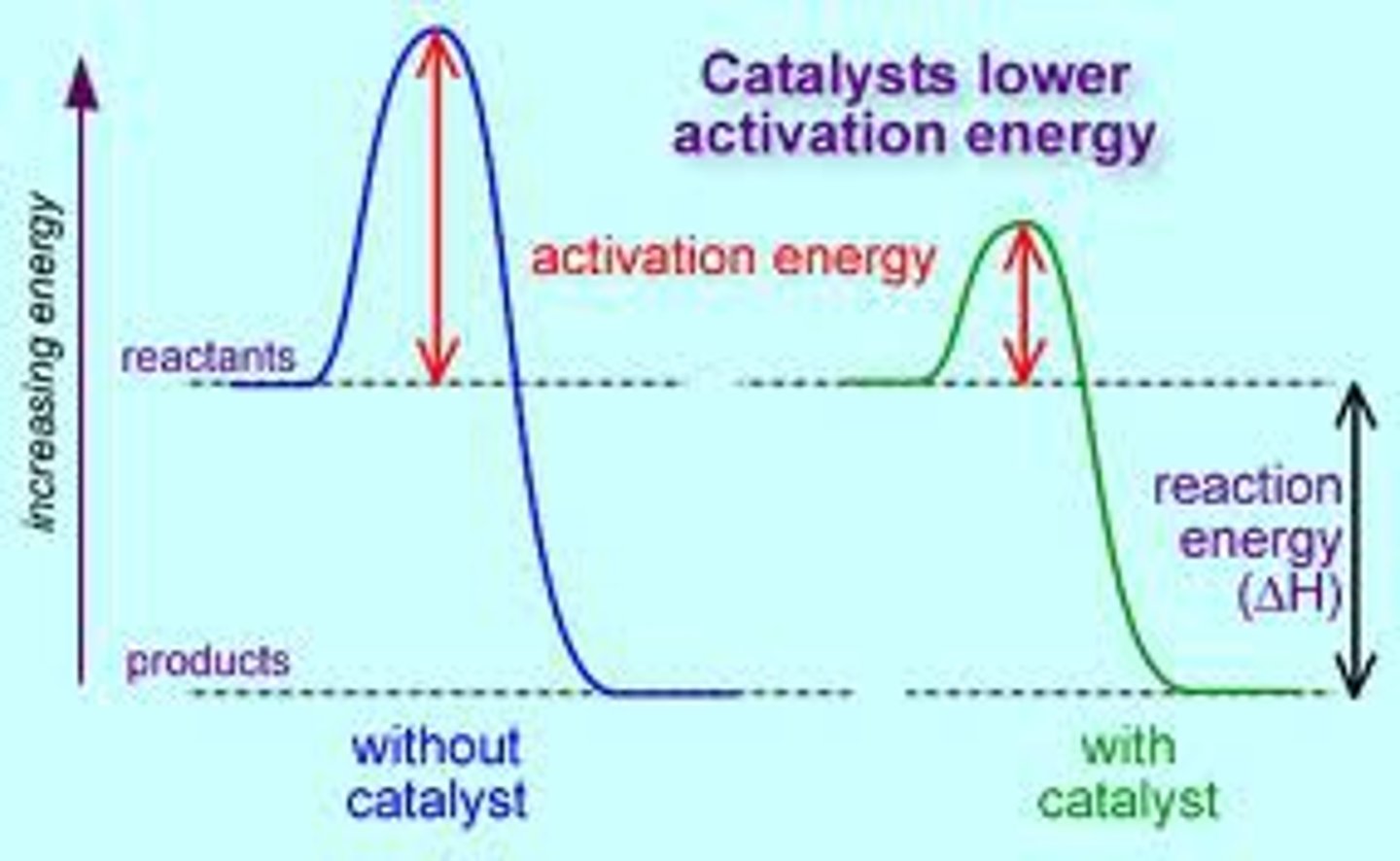
Activation Energy
- Minimum energy needed for chemical reaction to occur.
Definition of Rate
- 2A + B -> C
- Negative sign in front of the rate expression for the reactants
- Expressed in moles per liter per sec (mol/ L *s) or molarity per sec (M/ s)
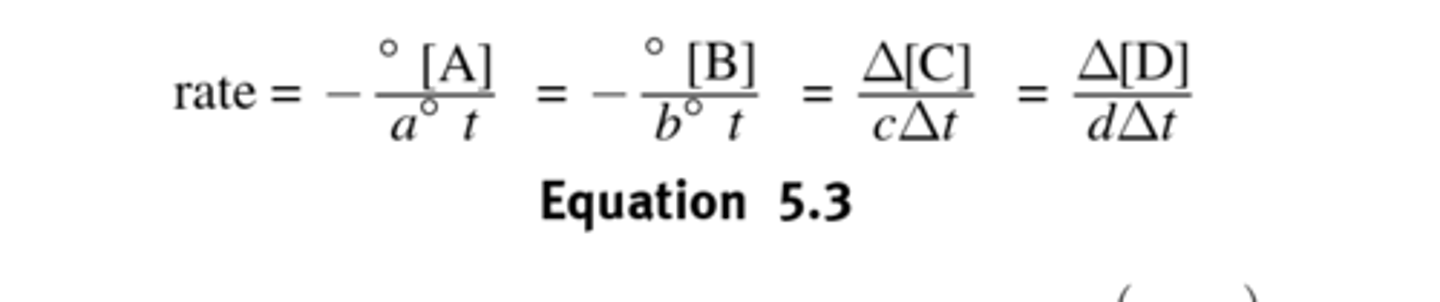
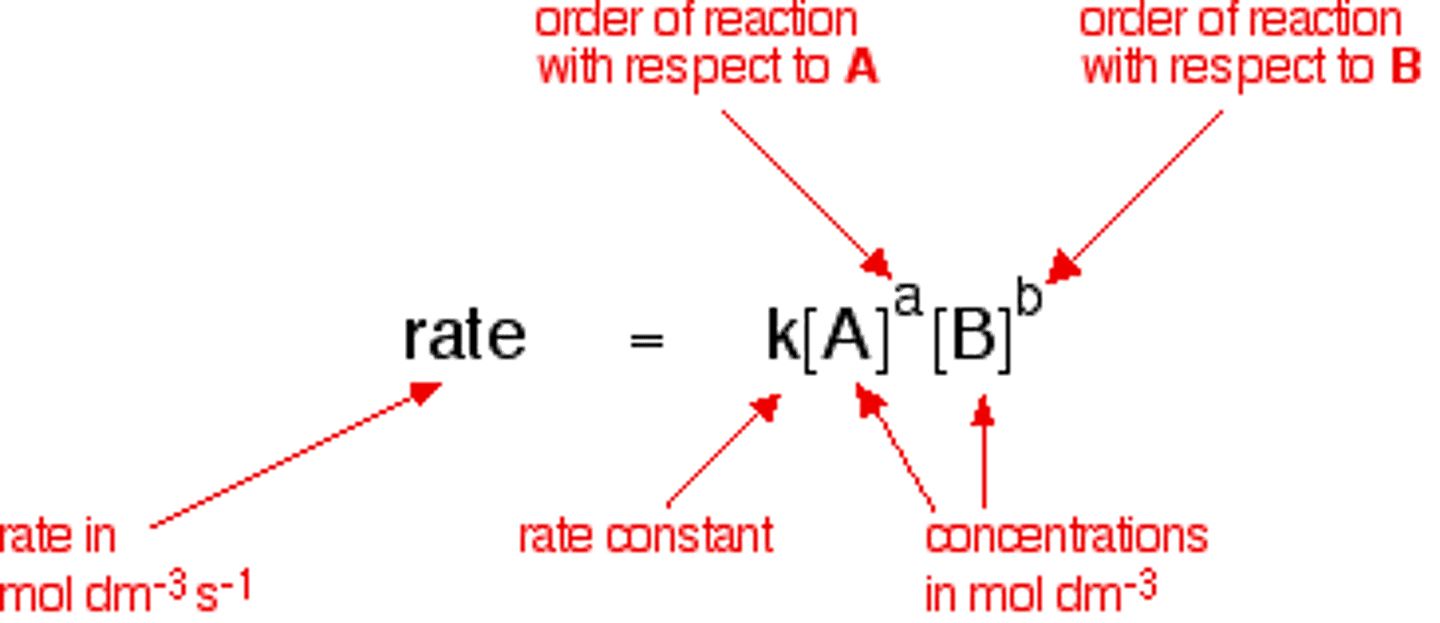
Rate Law
- Does Not depend on the concentration of products
- The exponents are unrelated to stoichiometric coefficients, so the amount of A consumed is not equal to the amount of B consumed.
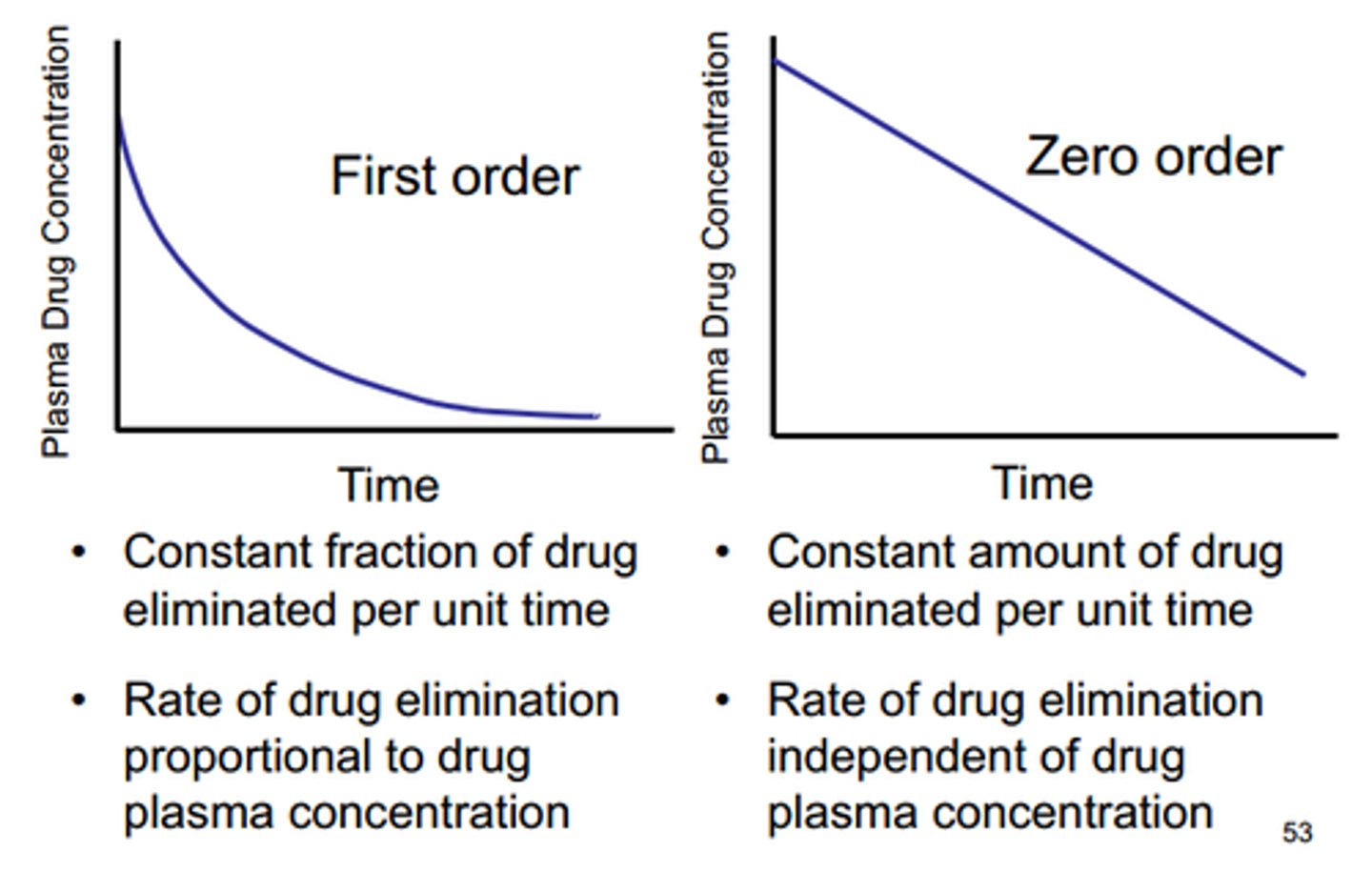
Zero-Order Reaction
- Rate of formation of product is independent of changes in concentrations of any of the reactants.
- k has units of M/s
- k = - slope
- Temperature and the addition of a catalyst are the only factors that can change the rate of a zero- order reaction.
- addition of catalyst = lowers the activation energy = inc. k value
First order Reaction
- Directly proportional to only one reactant
- k has units of s^-1
- Rate doubles as concentration doubles
- ex) reactive decay
- Concentration vs. Time results in a non-linear graph
- Formation of product is dependent on the concentration of the reactant.
- slope of ln [A] vs. time is -k
![<p>- Directly proportional to only one reactant</p><p>- k has units of s^-1</p><p>- Rate doubles as concentration doubles</p><p>- ex) reactive decay</p><p>- Concentration vs. Time results in a non-linear graph</p><p>- Formation of product is dependent on the concentration of the reactant.</p><p>- slope of ln [A] vs. time is -k</p>](https://knowt-user-attachments.s3.amazonaws.com/eb753a81-42d6-4448-8ed5-93396c28ef85.jpg)
Second order Reaction
- Proportional to either the concentration of two reactants or to the square of the concentration of a single reactant.
- k has units of M^-1s^-1
- Concentration vs. Time results in a non-linear graph
- Formation of product is dependent on the concentration of the reactant.
- slope of 1/ [A] vs. time is k
![<p>- Proportional to either the concentration of two reactants or to the square of the concentration of a single reactant.</p><p>- k has units of M^-1s^-1</p><p>- Concentration vs. Time results in a non-linear graph</p><p>- Formation of product is dependent on the concentration of the reactant.</p><p>- slope of 1/ [A] vs. time is k</p>](https://knowt-user-attachments.s3.amazonaws.com/649d37dc-a465-4125-bac8-33f35a8f7e9e.jpg)
Dynamic Equilibrium and reversibility
- The rate of the forward reaction equals the rate of the reverse reaction, entropy is at a maximum.
- Links to the concept of thermodynamics and kinetics.
Reaction Quotient
- At any point in time during a reaction, we can measure the concentrations of all of the reactants and products and calculate the reaction.
- Qc < Keq ; Delta G < 0; forward reaction
- Qc > Keq; Delta G forward > 0; reverse reaction
- Qc = Keq; Delta G = 0; dynamic equilibrium
- Kc >> 1 the equilibrium mixture will favor products over reactants.
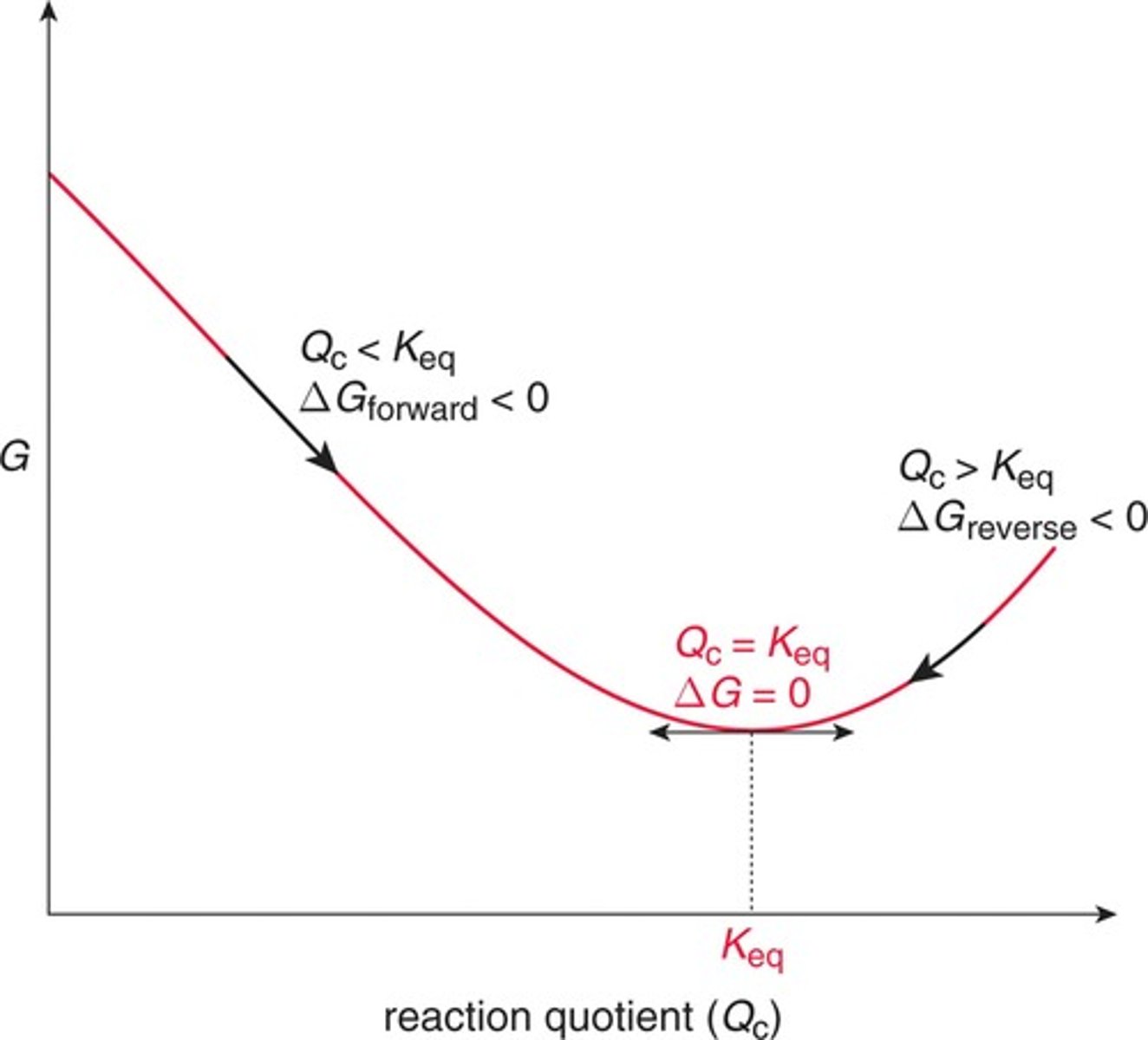
Keq Rules
- Characteristic of a particular reaction at a given temp; the equilibrium constant is temperature dependent.
- The larger the Keq, the farther to the right the equilibrium position
- if the equilibrium constant for a reaction written in one direction is Keq, the equilibrium constant for the reverse reaction is 1/ Keq .
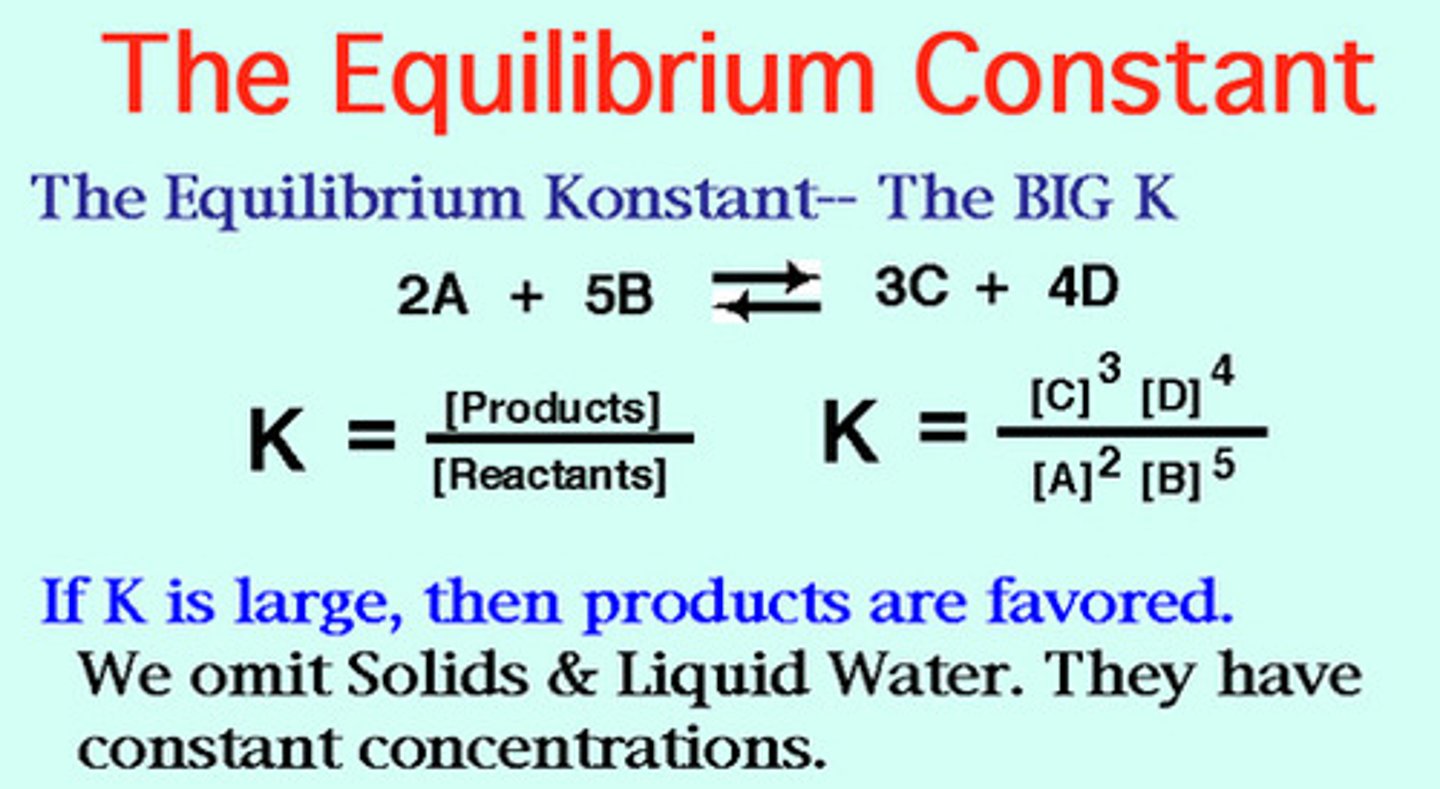
Enthalpy
- Delta H > 0; Endothermic; Heat act as a reactant
- Delta H < 0; Exothermic, Heat act as a product
- Delta H = heat (Q) under constant pressure.

The reversible reaction
- Delta H > 0; Endothermic; Heat act as a reactant
- Delta H < 0; Exothermic, Heat act as a product
- Always toward whichever side has the lowest total number of moles of gas.
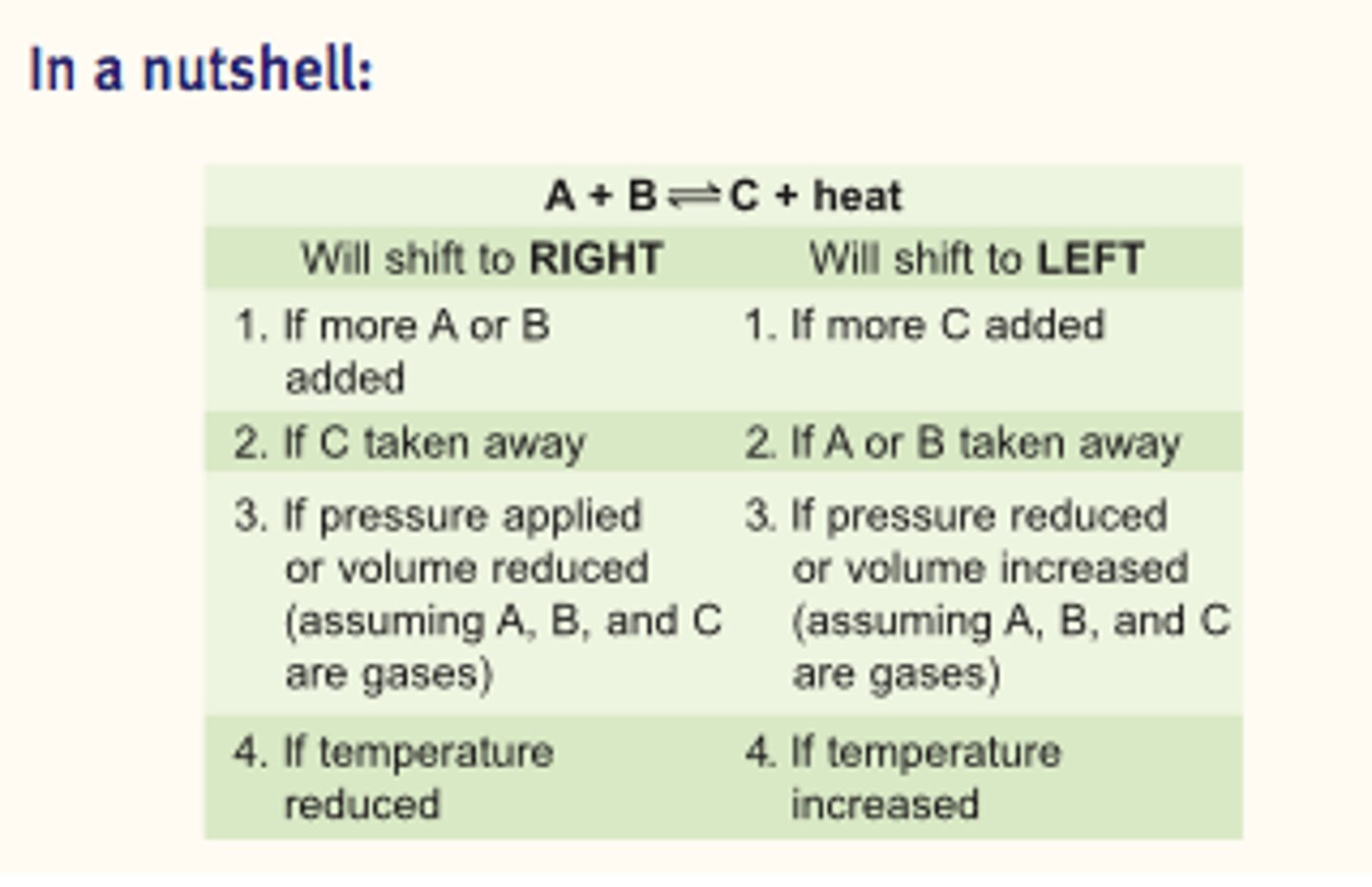
Kinetic and Thermodynamic Control
- Lower temperature: Kinetic Product; Forms faster
- Higher temperature: Thermodynamic Product; free energy is much lower; greater stability; more negative Delta G
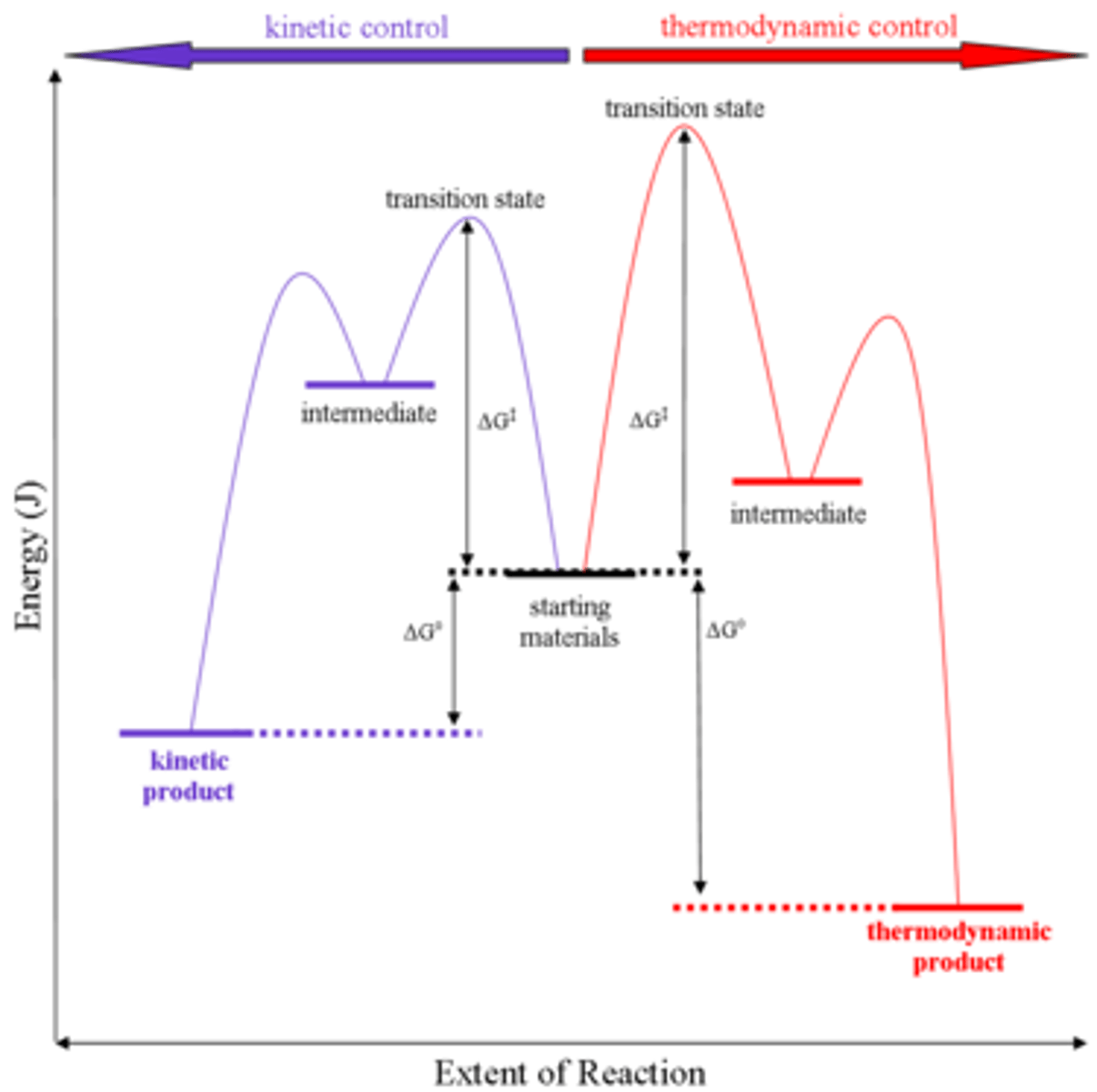
Average Kinetic Energy
- Directly proportional to the temperature of a gas in kelvins.
- Molecules are elastic and thus do not result in loss of energy.
- AVG kinetic energy of any gas as a whole is the same at a given temperature
Ka
- ratio of products to reactants
- Greater than 10^-7, solution contains more H+ which makes it acidic.
First Law of Thermodynamics
- Energy is never created nor destroyed; simply changes from one form to another.
- Delta U: change in internal energy of the system
- Q: heat added to the system
- W: Work done on the system
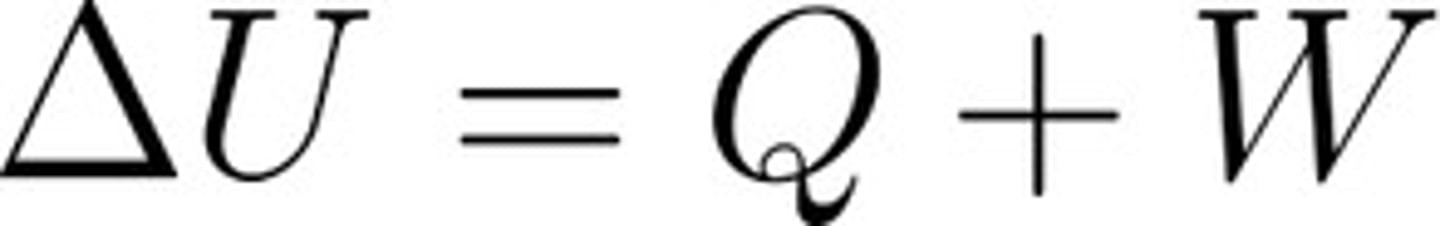
Isothermal
- No change in temperature; Delta U = 0, Q = W
- Temperature and the internal energy of the system is constant throughout.
- Hyperbolic curve on a pressure-volume graph (P-V graph)
- Work is represented by the area under the graph, but also the heat that entered the system.
-
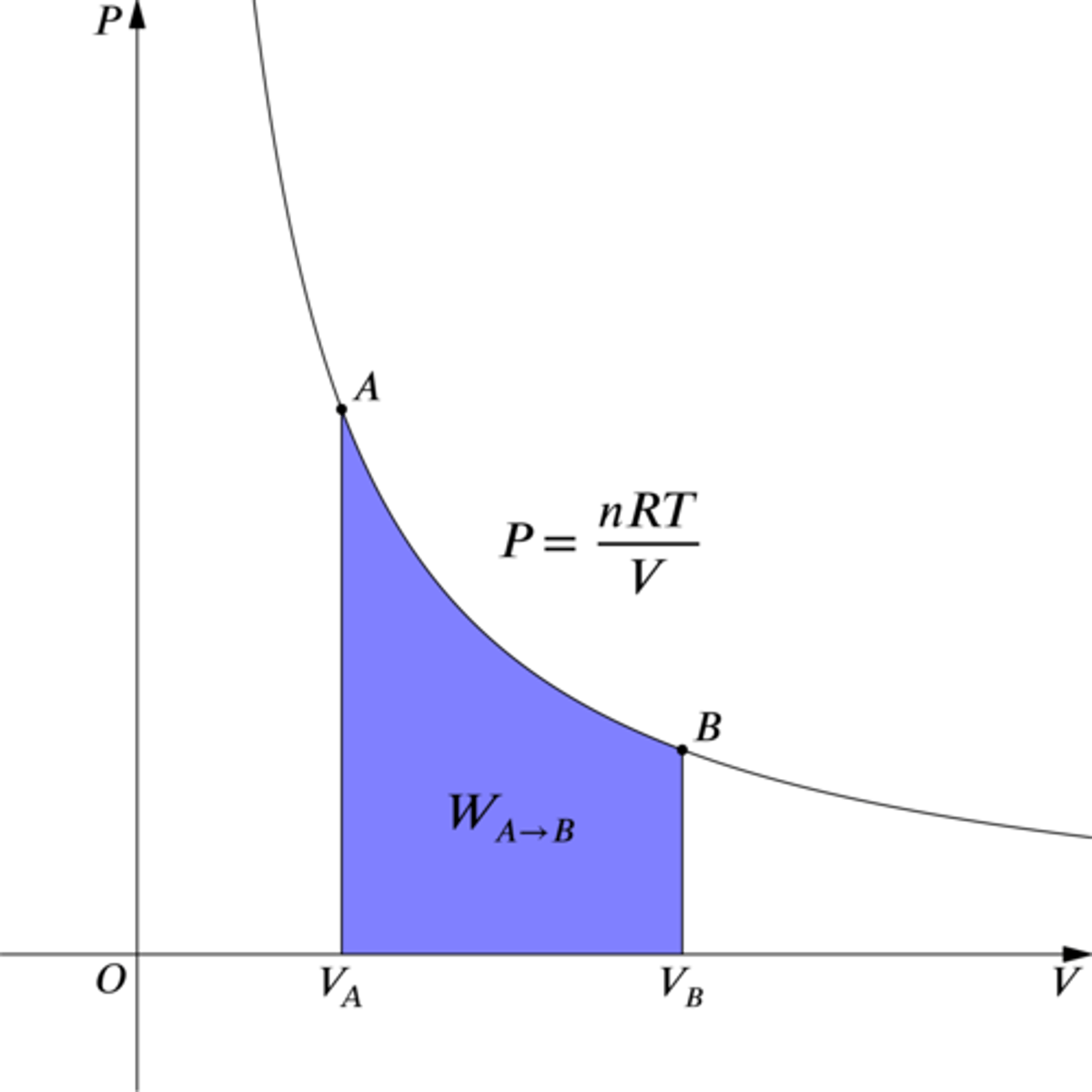
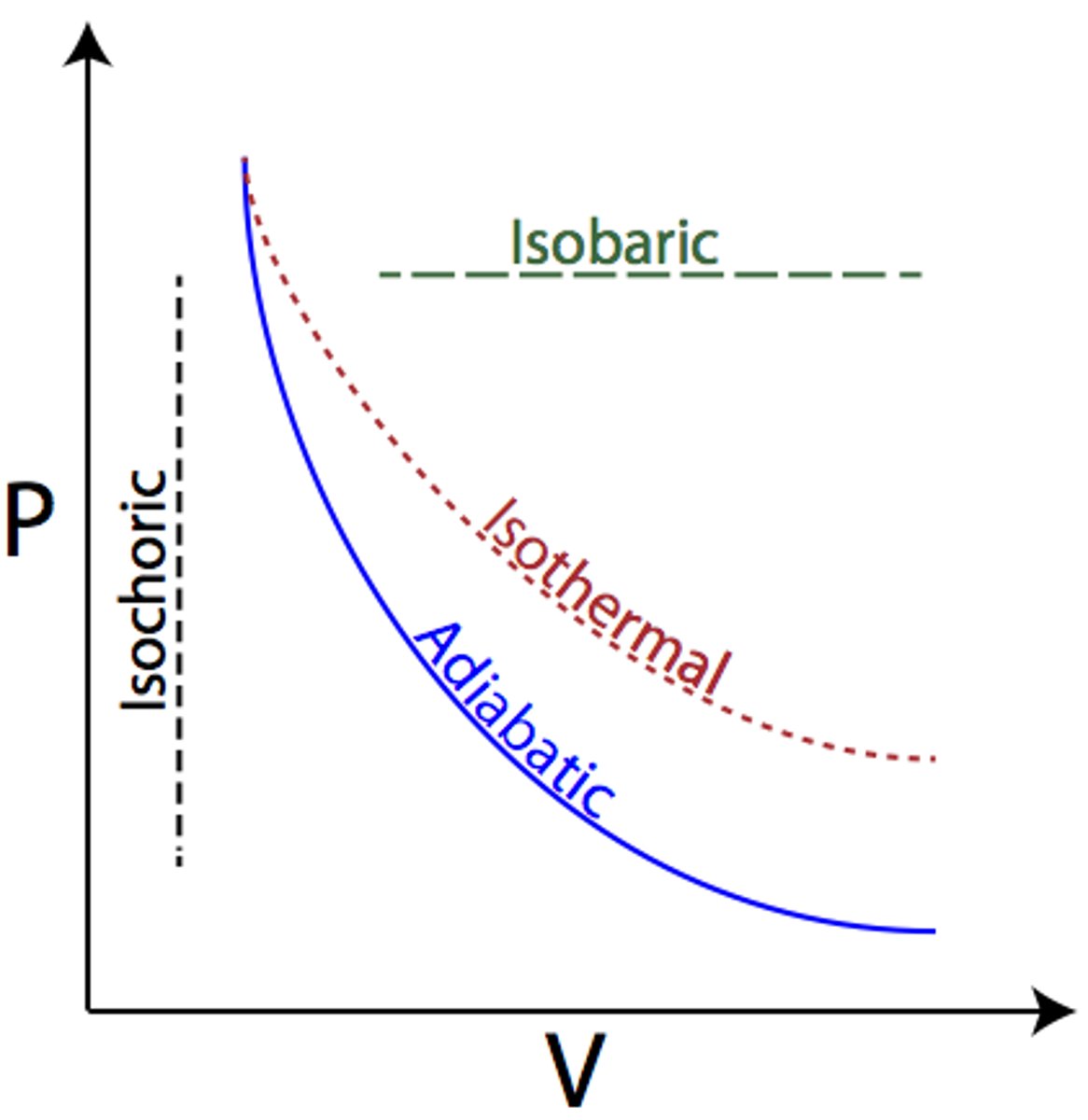
Adiabatic
- No heat exchange; Q = 0, Delta U = -W
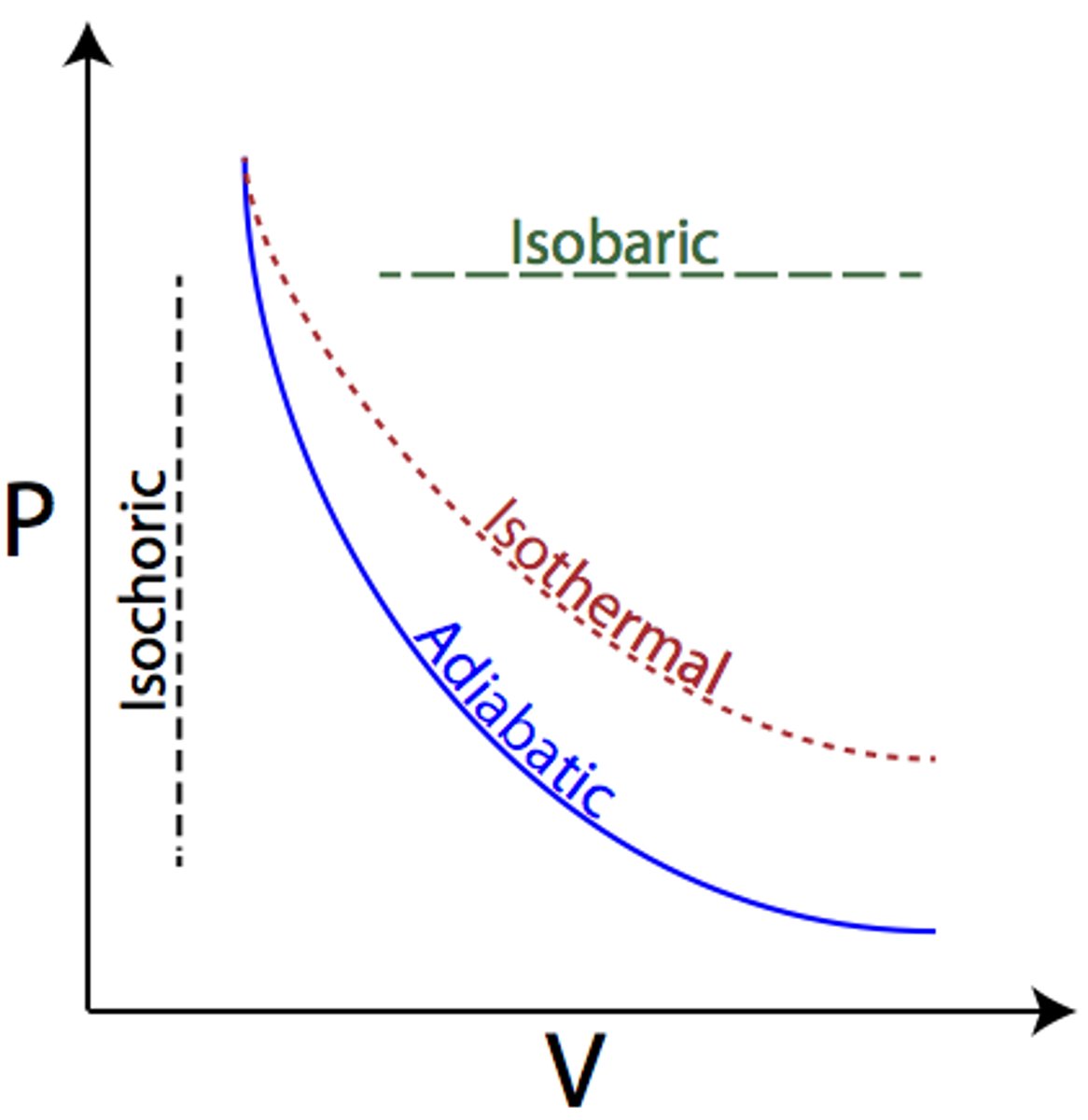
Isobaric
- No change in pressure, line appears flat in a P-V graph
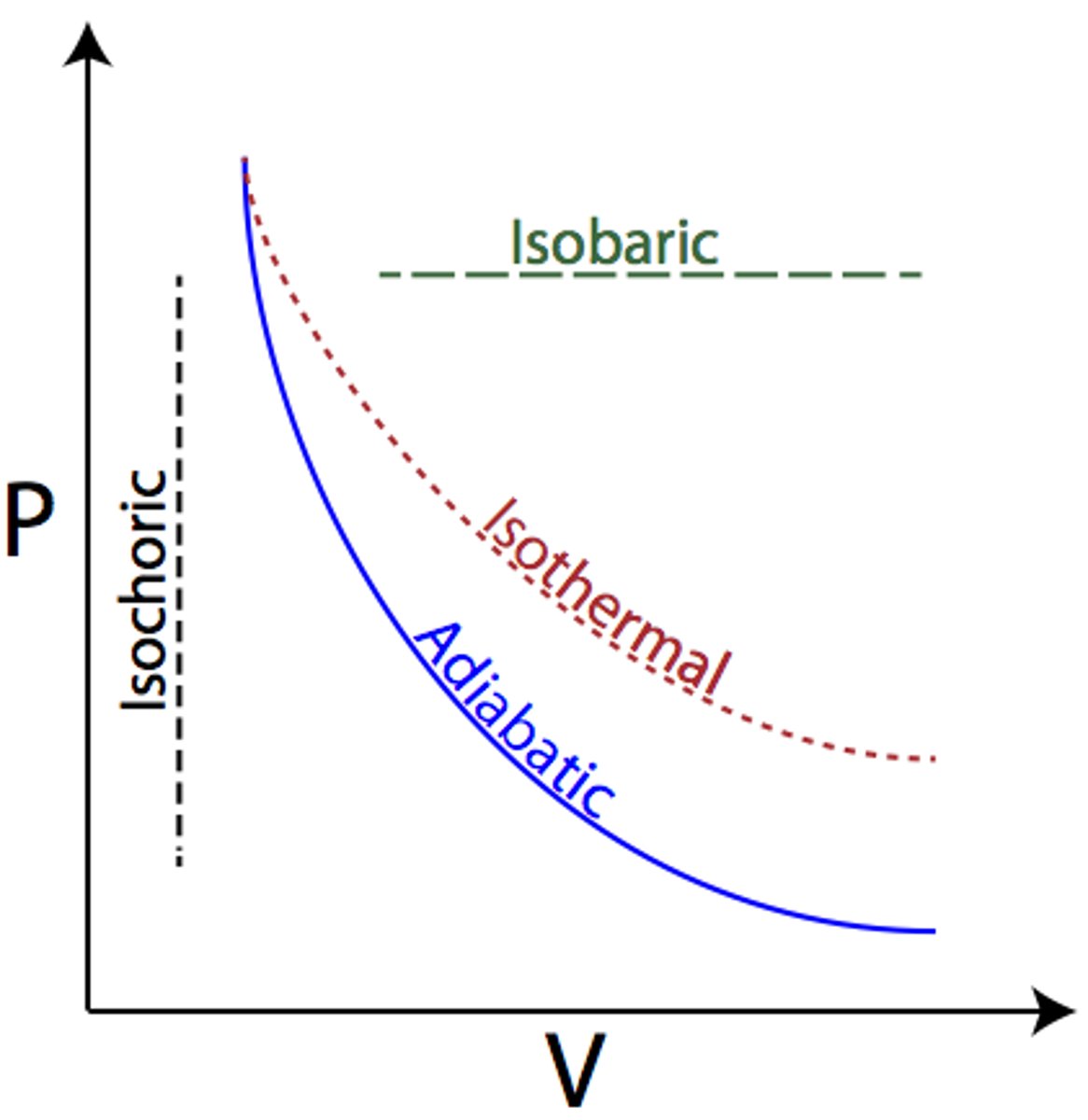
Isovolumetric / isochoric
- No change in volume
- W = 0, Delta U = Q
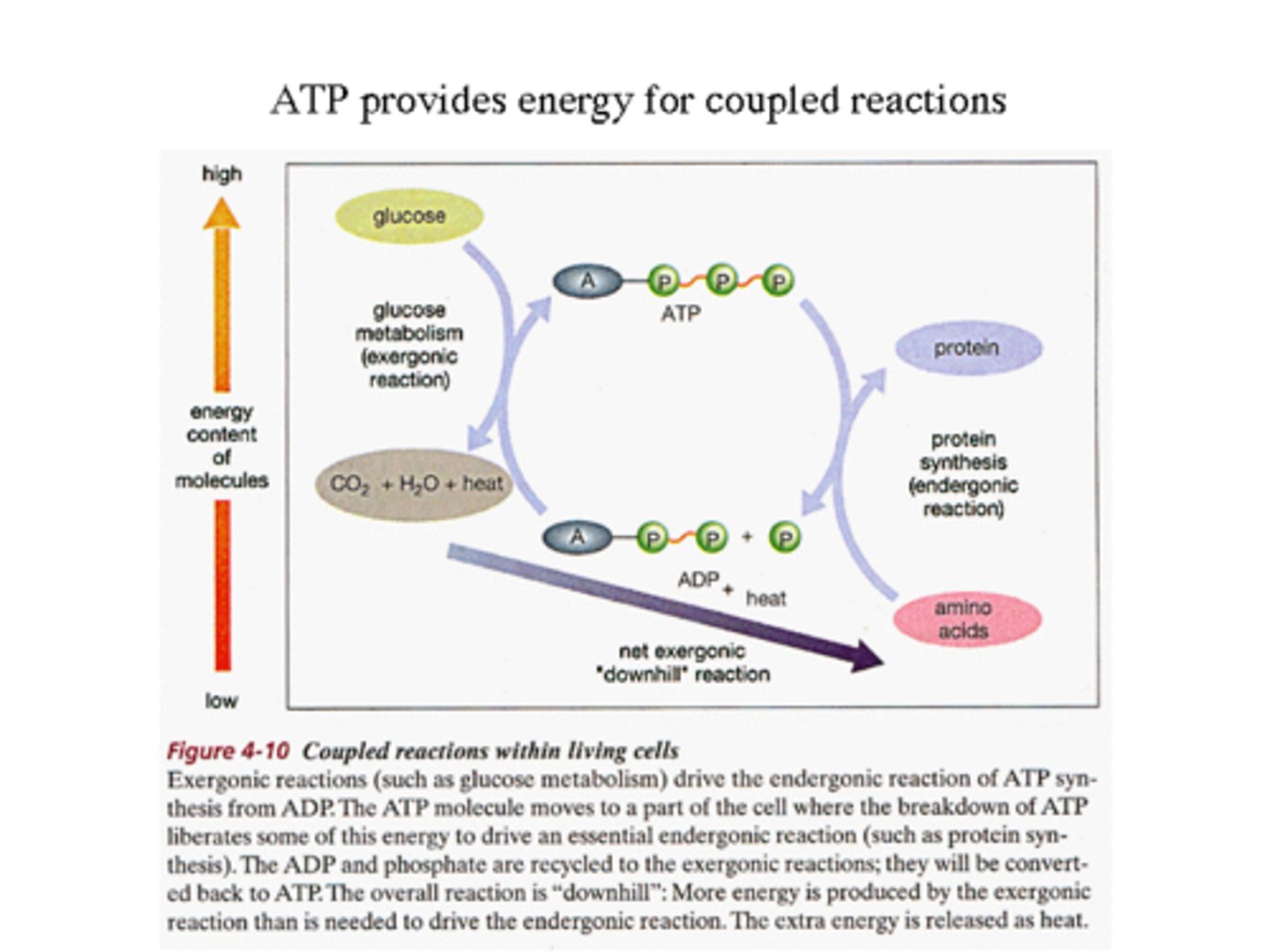
Coupling Reactions
- A common method for supplying energy for non spontaneous reactions is by coupling non spontaneous reactions to spontaneous
- Combustion of glucose is exergenic; the formation of peptide bonds is endergenic.
- Energy from the combustion can be stored in the peptide bonds in GTP, which are then lysed to provide the energy for forming peptide bonds.
State Functions
- Describe the system in an equilibrium state
- Pressure, density, temperature, volume, enthalpy, internal energy, Gibbs free energy, entropy.
Standard Conditions
- 25 C or 298 K
- 1 atm
- 1 M concentration
- used for kinetics, equilibrium, thermodynamics problem, electrochemical cell voltage
Standard Temperature and Pressure (STP)
- used for ideal gas calculations
- 273 K or 0 C
- a mole of ideal gas = 22.4 L

Ideal Gas
- Individual volume and intermolecular forces are negligible
- No volume or intermolecular forces between molecules in an ideal gas environment
Real Gases
- Deviate from ideal behavior under high pressure ( low volume) and low temperature conditions.
Gas Pressure units
1 atm = 760 mmHg = 760 torr = 101.325 kPa

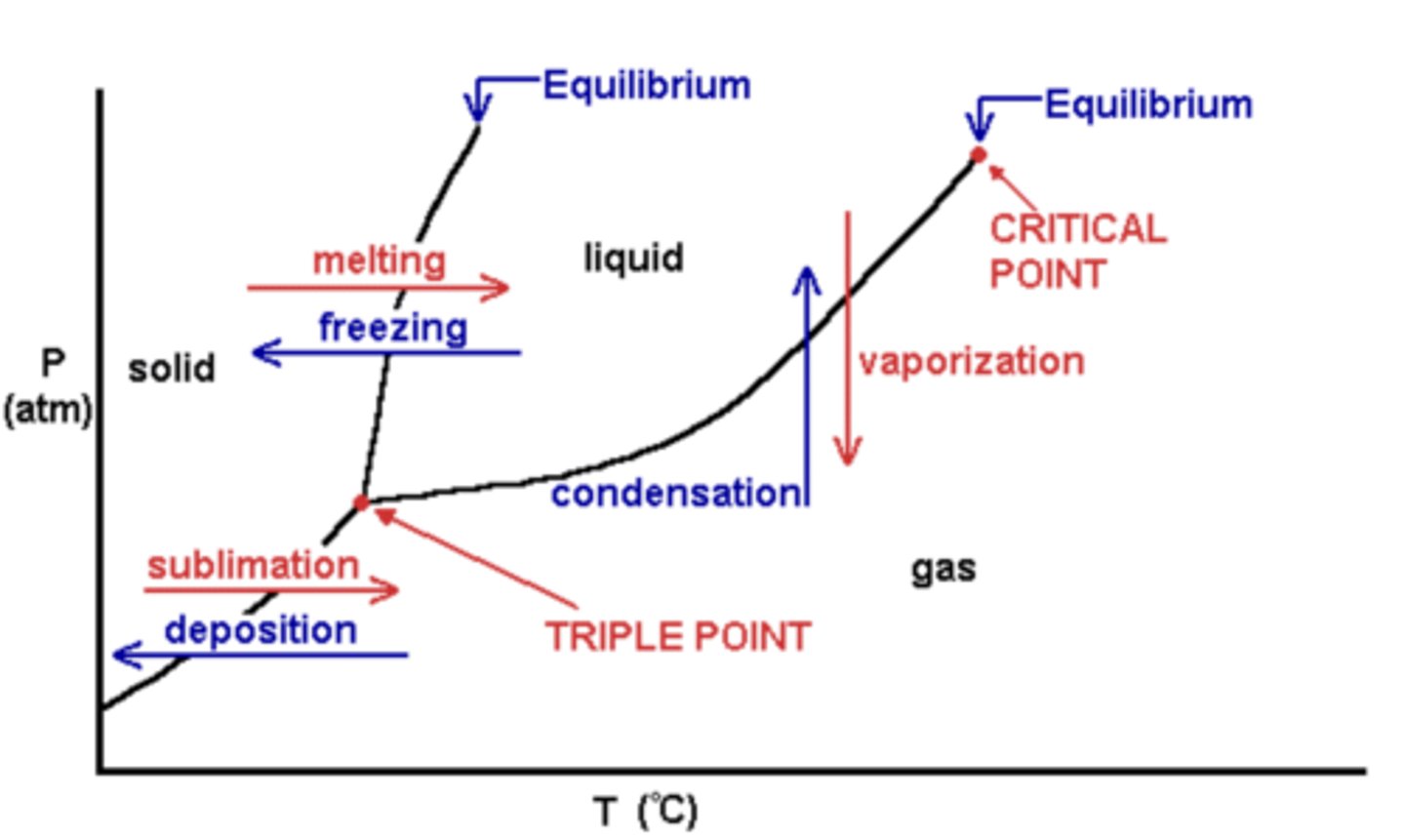
Triple point
- Combination of pressure and temperature at which 3 phases are at equilibrium
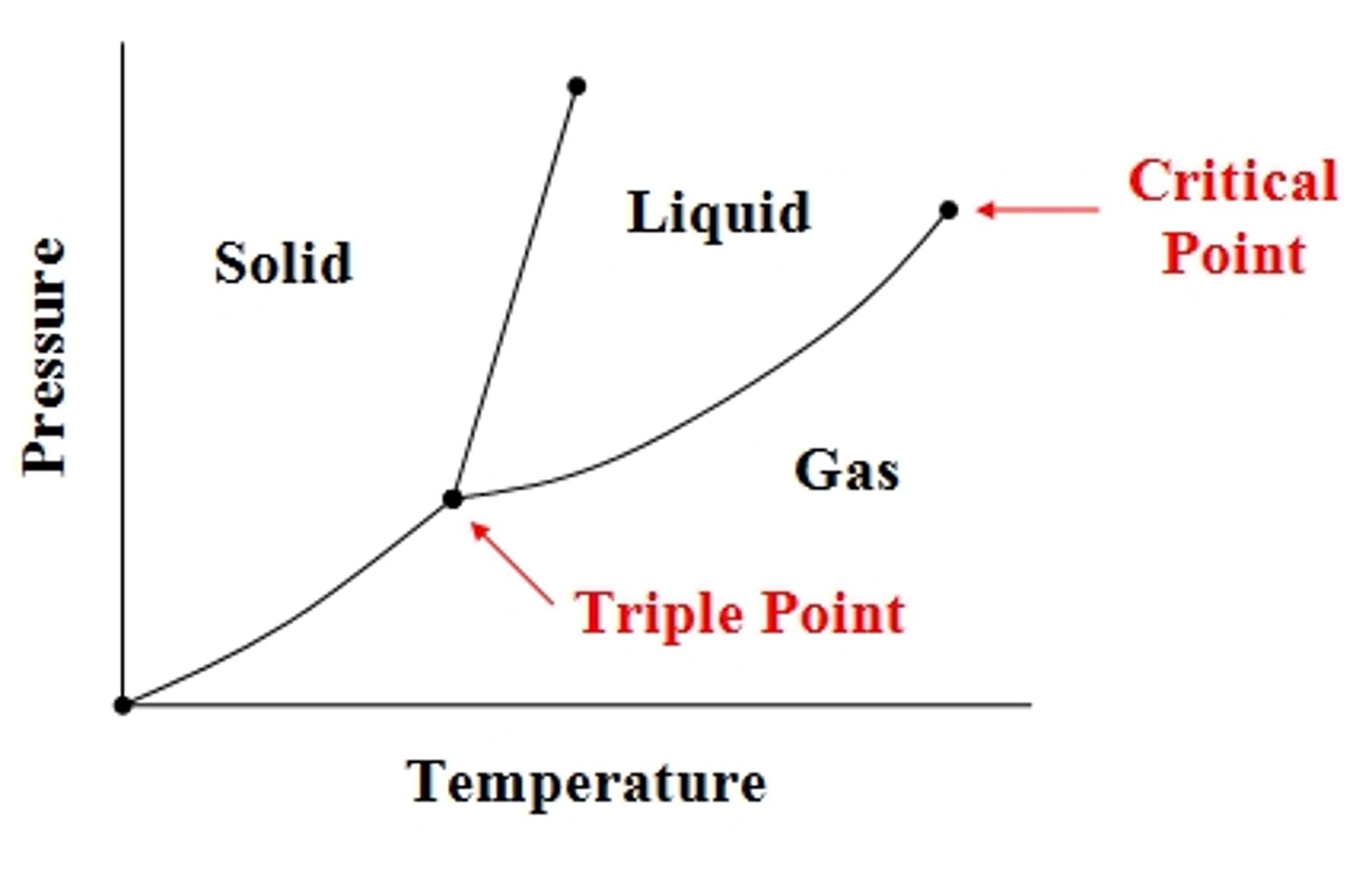
Critical Period
- Temperature and Pressure where liquid and gas are indistinguishable and heat of vaporization is zero
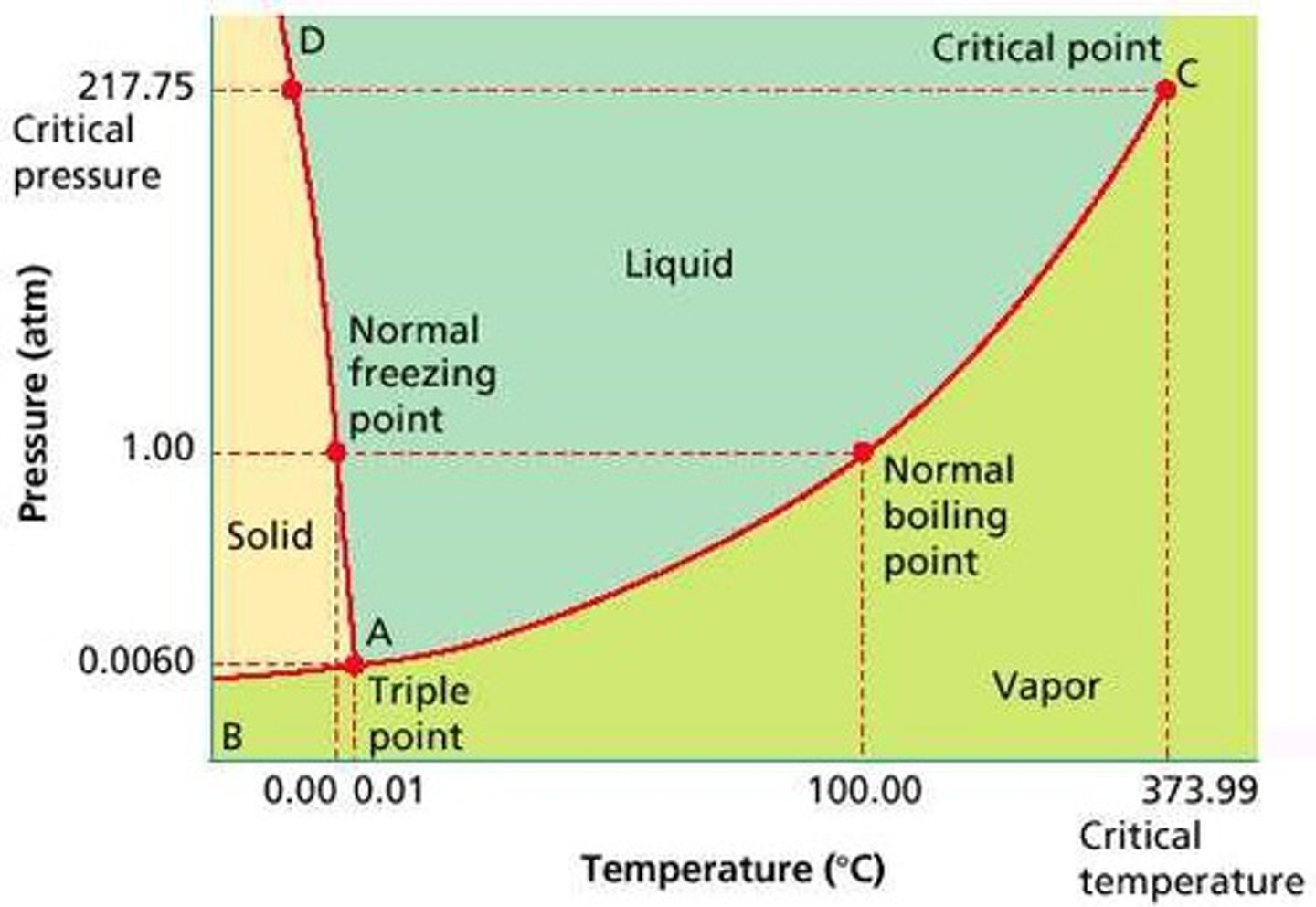
Phase Diagram
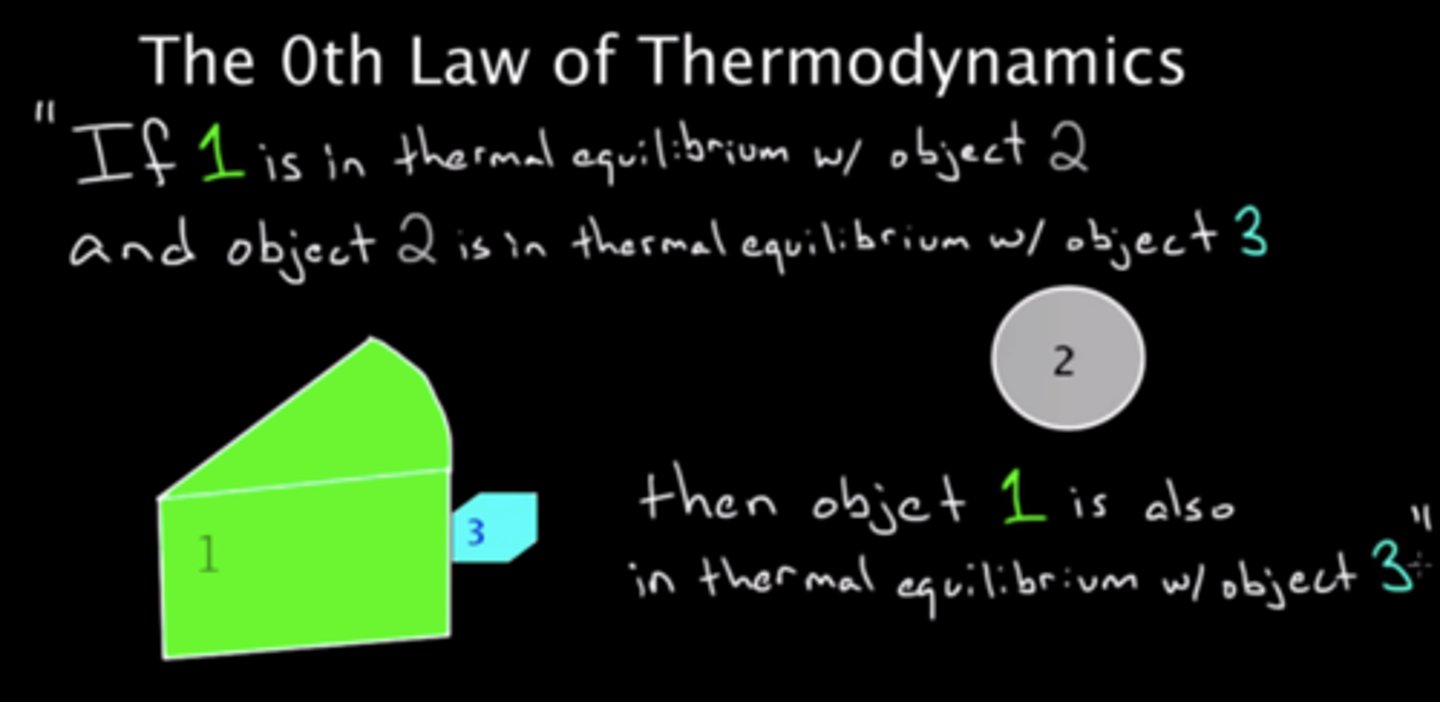
Zeroth Law of Thermodynamics
- Objects are in thermal equilibrium only when their temperatures are equal
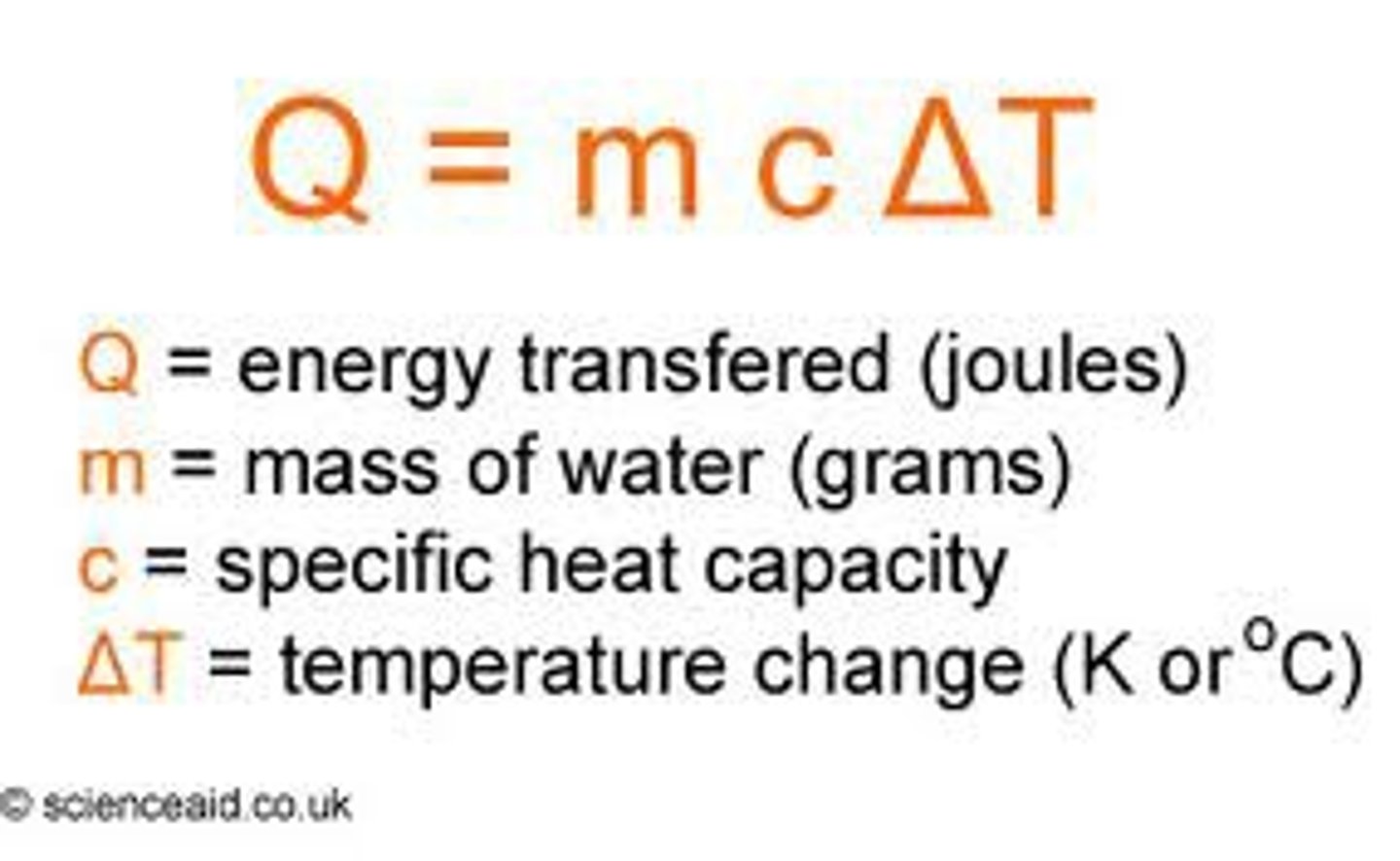
Heat Transfer Equation
- Heat transfer ( no phase change)
- Heat capacities: mass times specific heat ( m*c)
- sweating cools you down by giving energy to the sweat to make it evaporate
- Specific heat of water ( in calories) = 1 cal/ g*k
Latent Heat
- Heat transfer used during phase changes
- Enthalpy of an isothermal process
- units cal/g
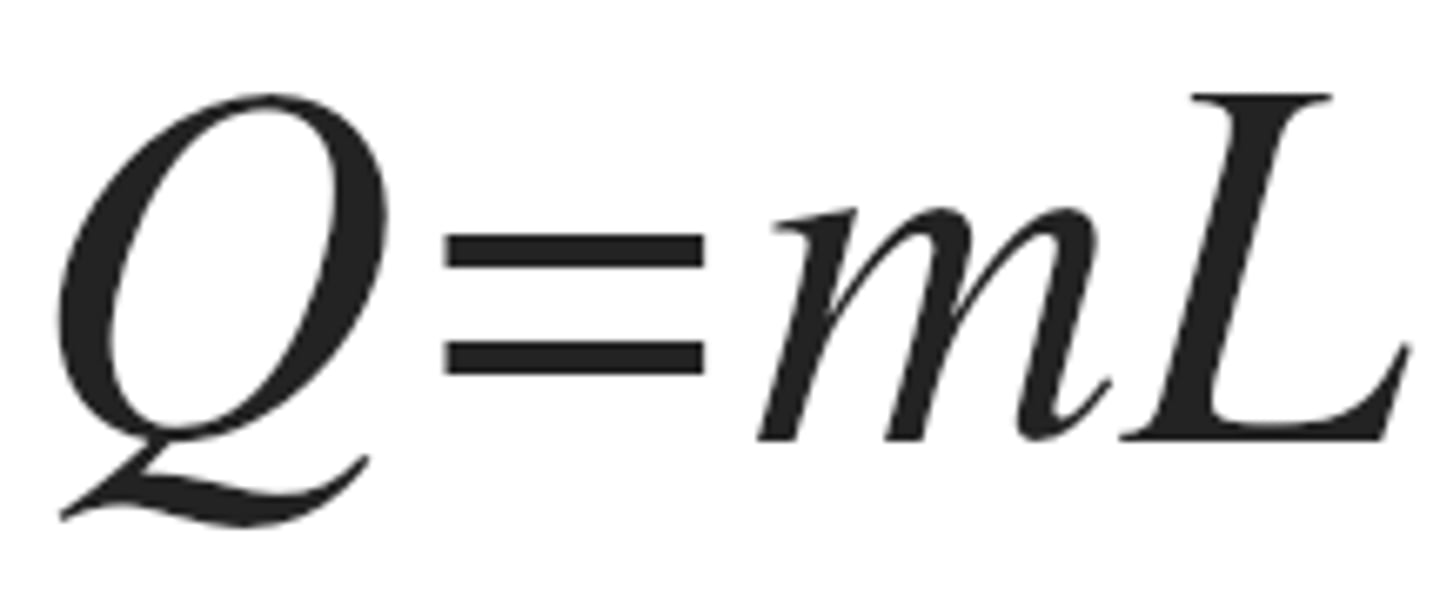
Second Law of Thermodynamics
- Energy spontaneously disperses from being localized to becoming spread out.
- Q is heat that is lost or gained
- T is temperature in Kelvin
- units: J/ (mol * K)
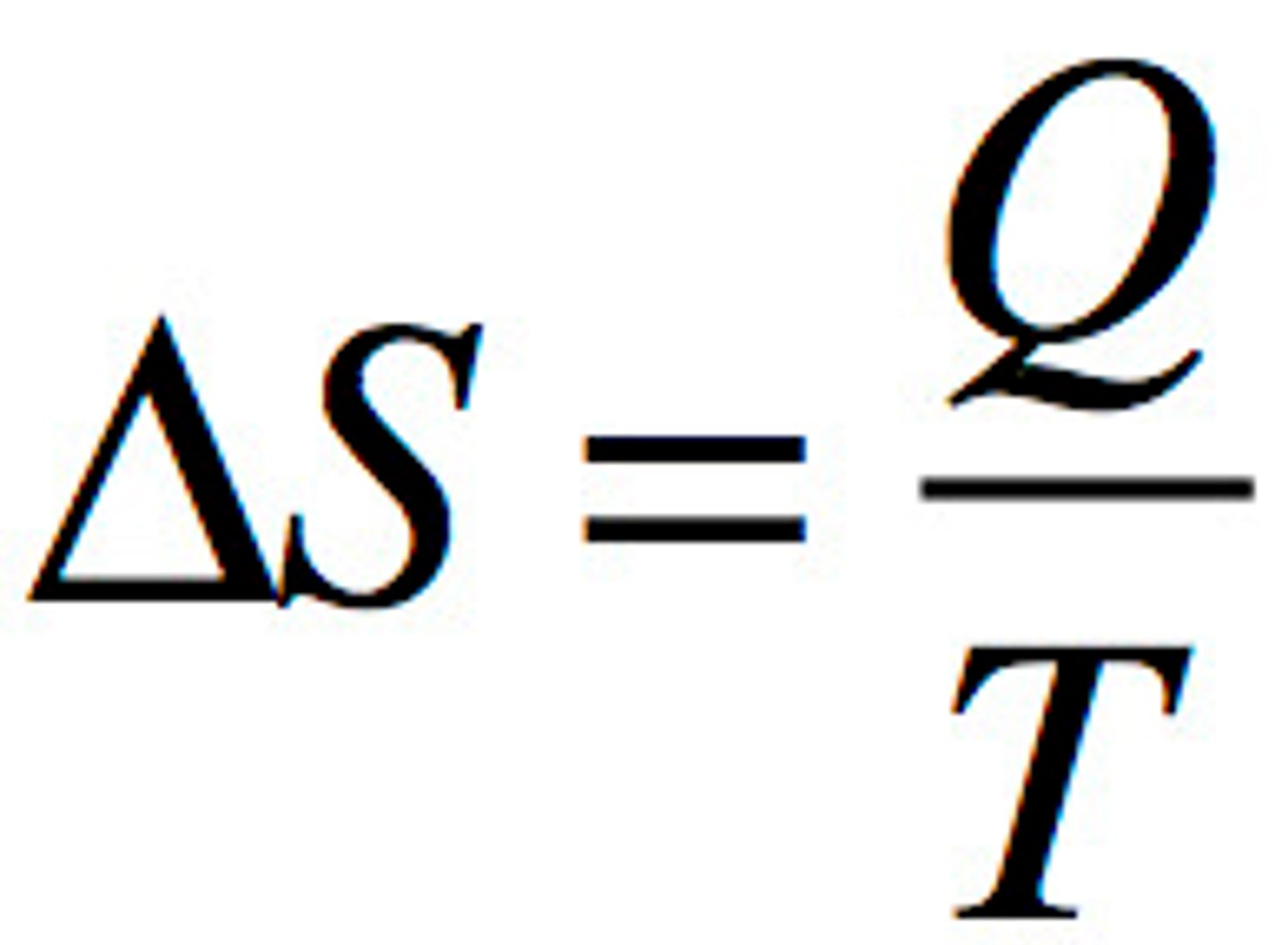
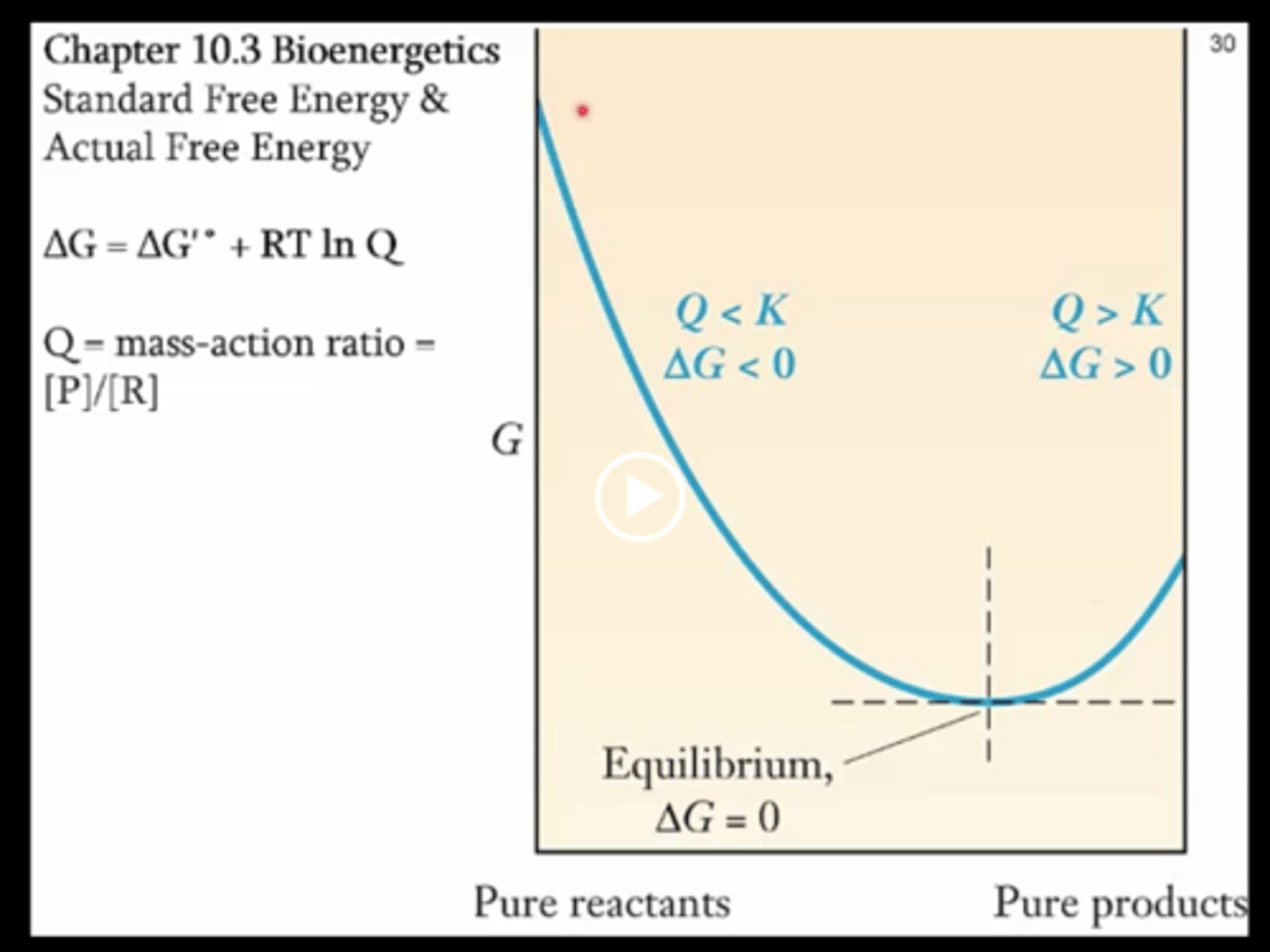
Standard Gibbs free energy from reaction quotient
Also = RT Ln Q/ Keq
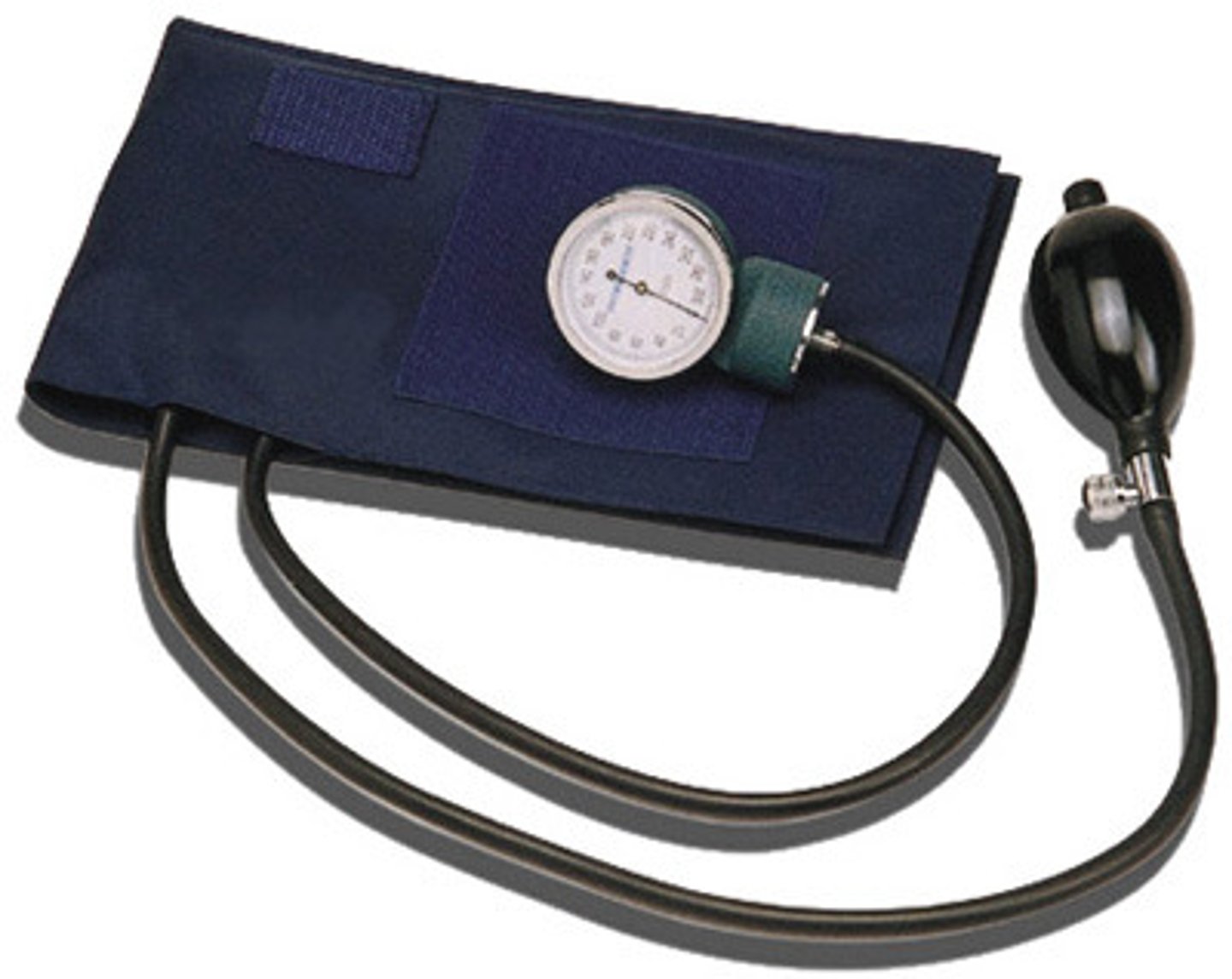
Sphygmomanometer
- Medical device that measure blood pressure
- unit of measurement is mmHg
- Normal : 120 mmHg systolic and 80 mmHg diastolic (120/80)
- Hypertension: > 140 mmHg systolic or > 90 mmHg diastolic
- Systolic: Heart Contraction
- Diastolic: Heart relaxation
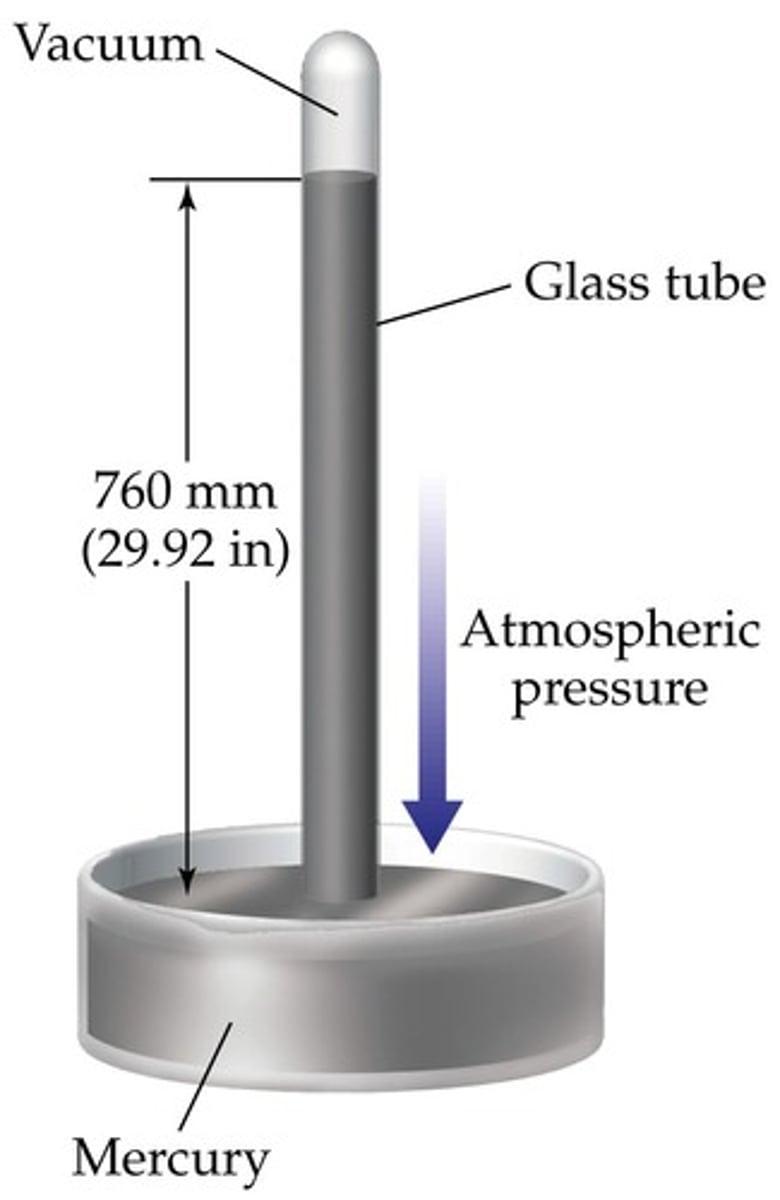
Atmospheric Pressure
- When the external air exerts a higher force than the mercury, the column rises
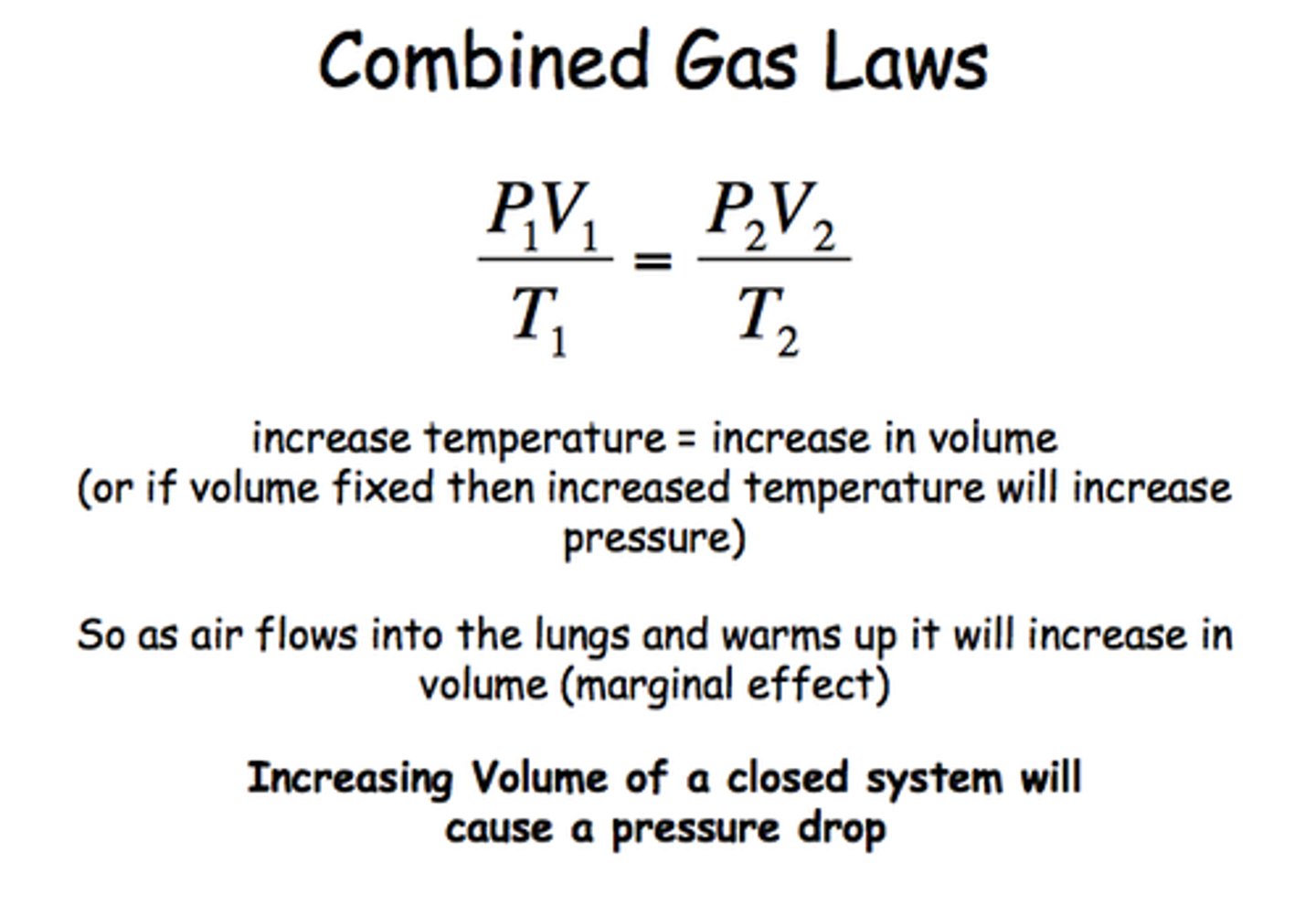
Combined Gas Law
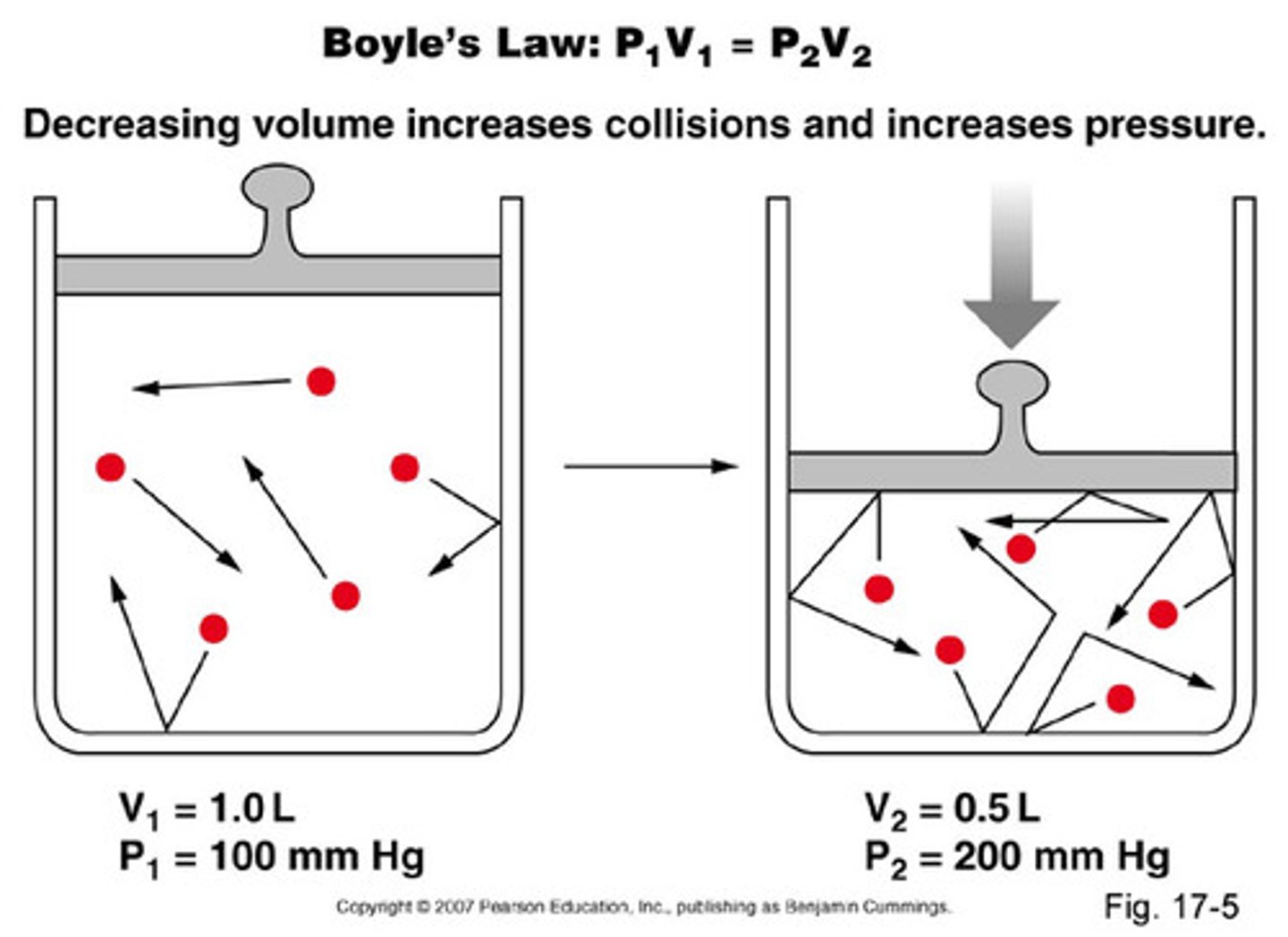
Boyle's Law
- Temperature and the number of moles are held constant.
- Inverse relationship between pressure and volume.
- As pressure increases, volume decreases
Charle's Law
- At constant pressure, the volume of a gas is proportional to its absolute temperature
- V1/T1 = V2/T2
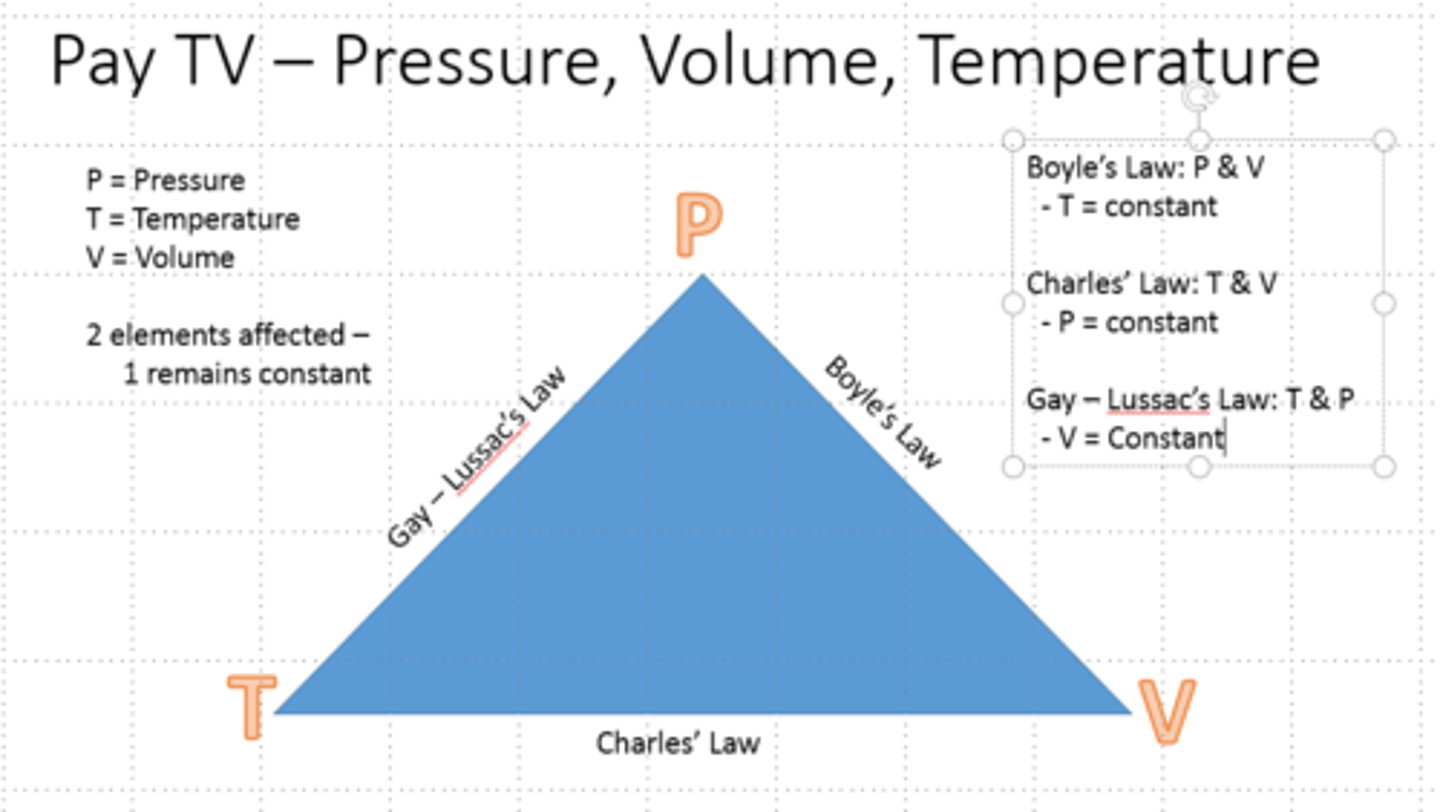
Gay-Lussac's Law
- Relates pressure to temperature
- similar to charle's law : V/ T
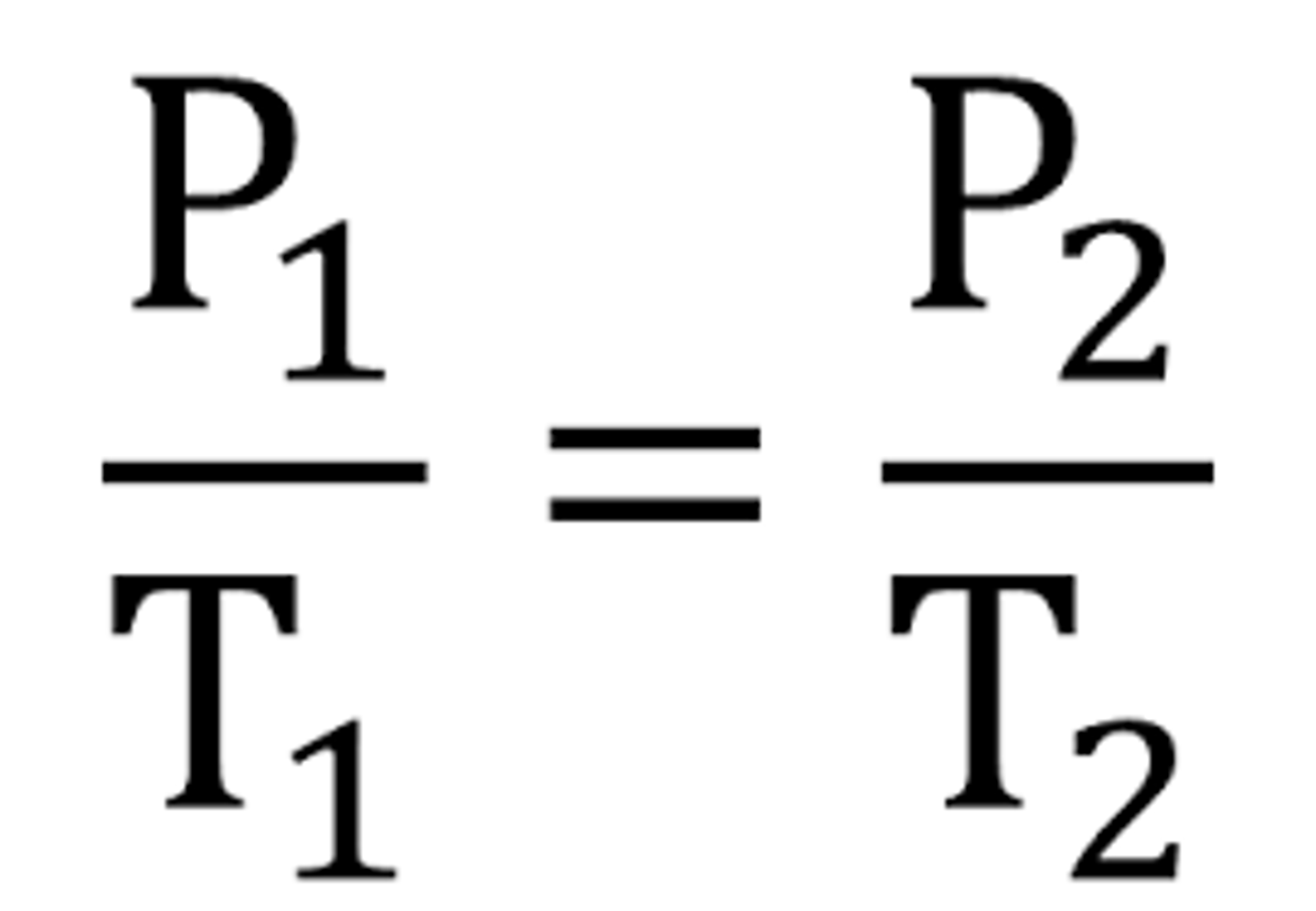
Dalton's Law of partial Pressure
Total Pressure of a gaseous mixture is equal to the sum of the partial pressure of the individual components.