Biology - Unit 1: Chemistry of Life
1/23
Earn XP
Description and Tags
flashcards for Unit 1 of AP Biology; updated as of 2026
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
covalent bonds
bonds between atoms to fill their valence electron shells
nonpolar
equal sharing of electrons - no charge
polar
unequal sharing of electrons - has a charge
hydrophilic
dissolves in water
hydrophobic
doesn’t dissolve in water
hydrogen bonds
attraction between oxygen and hydrogen due to the polarity of water molecules
evaporation
kinetic energy between water molecules on the surface releases some into the atmosphere
solvent
a substance that dissolves stuff
sphere of hydration
water molecules surround the substance they bond to
cohesion
water’s tendency to stick to itself - causes surface tension
adhesion
attraction between water molecules and other molecules - causes a meniscus and capillary action
isotopes
different forms of an element that has a different amoun of neutrons
atomic mass
mean mass of an element’s isotopes
orbitals
sets of electron shells represented by “n” in the Bohr model
octet rule
states that atoms are the most stable when tehy have 8 electrons in their valence (outermost) shell
cations
positive ions; lose electrons
anions
negative ions; gain electrons
covalent bonds
strong bond when two atoms share electrons
aliphatic hydrocarbons
linear chains of carbon
aromatic hydrocarbons
closed rings of carbon
isomers
molecules that have the same chemical composition but different structures
hydroxyl
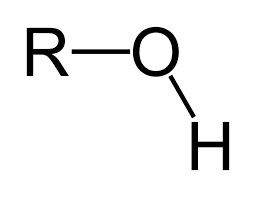
polar
methyl
R-CH3