Chem Chapter 1: Matter and Measurements
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
Chemistry
The study of the composition, properties, and interactions of matter.
Characteristics of a chemical change
Color change, temperature change, gas produced, forms precipitate
Mass
How much matter is in an object
Pure substance
Has a constant composition and properties throughout the sample. Consists of only one type of particle (atoms or molecules)
Compound
Substance made up of more than one type of atom
Mixture
A physical combination of two or more substances where each retains its individual properties. Mixtures can be either homogeneous or heterogeneous.
Homogenous Mixture
A mixture that has a uniform composition and properties throughout
Heterogenous Mixture
A mixture with an uneven composition throughout, where the individual components can be identified and separated.
Weight
The measure of the force exerted on an object due to gravity, commonly expressed in newtons or pounds.
Density
The mass per unit volume of a substance, often expressed in grams per cubic centimeter (g/cm³) or kilograms per liter (kg/L). Density is a physical property that indicates how much mass is contained within a given volume.
Accuracy
The degree to which a measured value matches the true or accepted value. Accuracy is important in ensuring reliable and valid results in measurements.
Precision
The consistency of repeated measurements or results, indicating how close the measurements are to each other, regardless of their accuracy.
Deca
Ten da 10^1
Hecto
Hundred h 10²
Kilo
Thousand k 10³
Mega
Million M 10^6
Giga
Billion G 10^9
Tera
Trillion T 10^12
Deci
Tenth d 10^-1
Centi
Hundreth c 10^-2
Milli
Thousandth m 10^-3
Micro
Millionth µ 10^-6
Nano
Billionth n 10^-9
Pico
Trillionth p 10^-12
Fahrenheit to Celsius/Celsius to Fahrenheit

Celsius to Kelvin/Kelvin to Celsius
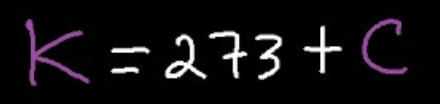
Volume Formula
Volume=Mass/Density
Density Formula
Density=Mass/Volume
Mass Formula
Mass=DensityxVolume