CHAPTER 2 -- pH, pKA, Ionization
1/20
Earn XP
Description and Tags
practice questions
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
21 Terms
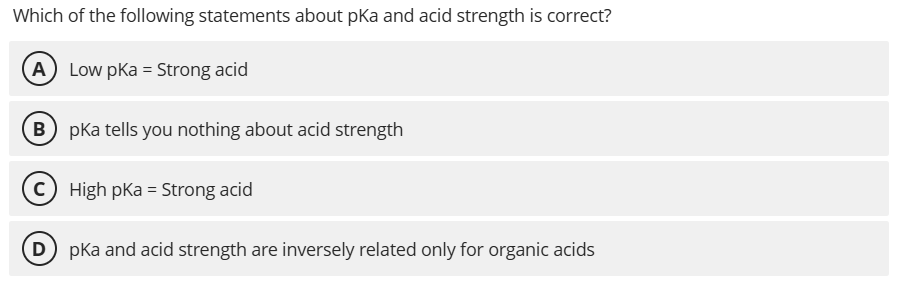
A. Low pKa = Strong acid
A low pKa indicates a strong acid because the acid readily donates its proton, resulting in a larger Ka and thus a smaller pKa value

B. pH = pKa + log([A⁻]/[HA])
The Henderson-Hasselbalch equation relates pH to pKa and the ratio of conjugate base to acid concentrations.
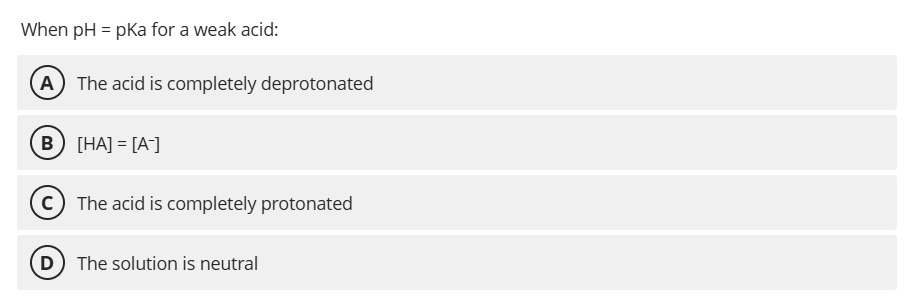
B. [HA] = [A⁻]
When pH = pKa, the concentrations of the protonated and deprotonated forms are equal, meaning the acid is 50% ionized.

B. Carboxylic acid > Thiol > Ammonium ion > Phenol
Lower pKa values indicate stronger acids, so the order follows: 4.5 < 8.5 < 9.2 < 10.0
True or False: Uncharged molecules generally cross cell membranes more readily than charged molecules.
True
uncharged molecules are more lipophilic and can pass through the hydrophobic core of cell membranes more easily than charged species

D. 100
Using Henderson-Hasselbalch: pH - pKa = 6.7 - 4.7 = 2, so log([A⁻]/[HA]) = 2, therefore [A⁻]/[HA] = 10² = 100.

B, C, and D
At pH 1, all groups with pKa values above 1 will be predominantly protonated (pH < pKa).
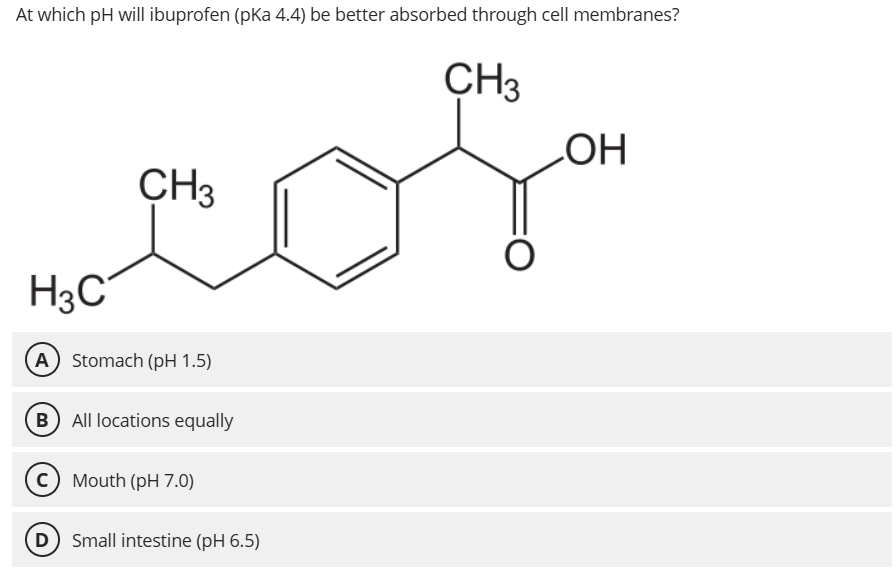
A. Stomach (pH 1.5)
At pH 1.5 (< pKa 4.4), ibuprofen is predominantly protonated and uncharged, facilitating membrane permeation

C. 1%
pH - pKa = 3 - 5 = -2, so [A⁻]/[HA] = 10⁻² = 0.01, meaning ~1% is deprotonated.

D. Increases by a factor of 100
At pH 4: ratio = 0.1; at pH 6: ratio = 10; the change is 10/0.1 = 100-fold increase.

C. Have a net negative charge
When pH > pI, the protein has a net negative charge due to the deprotonation of acidic groups
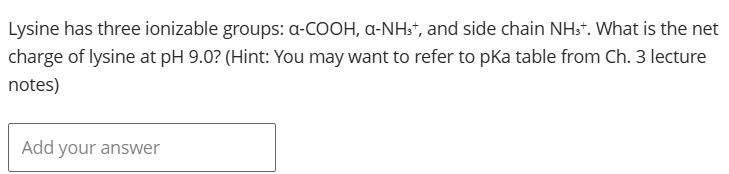
0.5
At pH 9.0: α-COOH is deprotonated (-1), α-NH₃⁺ is 50% protonated (+0.5), side chain NH₃⁺ is protonated (+1), giving net charge of +0.5.
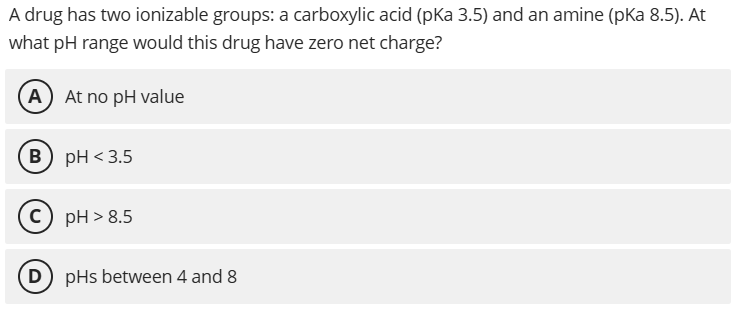
D. pHs between 4 and 8
Between pH ~4-8, the carboxylic acid is deprotonated (-1) and the amine is protonated (+1), yielding zero net charge. Note there is some rounding happening in the answer. The carboxylic acid would not be fully deprotonated at 4.0, but somewhere around 75% which we'll consider close enough.

D. Acetic acid (pKa = 4.7)
Buffers work best when pH is within ±1 unit of the pKa; acetic acid (pKa 4.7) is closest to pH 5.0.
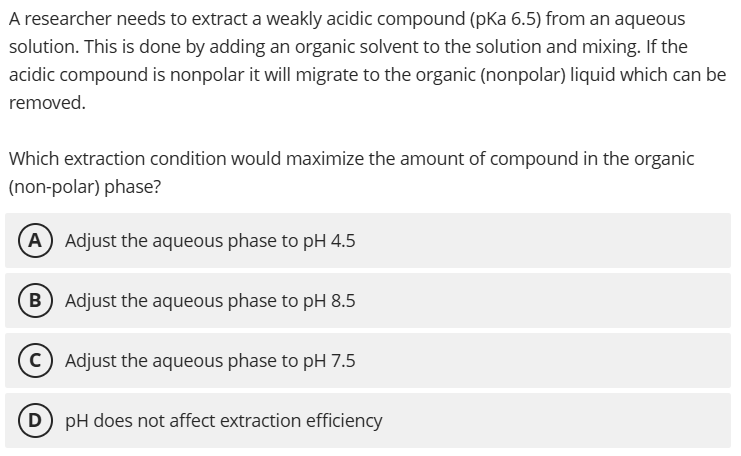
A. Adjust the aqueous phase to pH 4.5
At pH 4.5 (< pKa 6.5), the compound is predominantly protonated and uncharged, making it more soluble in the organic phase

D. Partial protonation of the histidine side chain facilitates cycling between protonated and deprotonated states
Near its pKa (6.0), histidine can readily switch between protonated and deprotonated forms depending on the environment or molecules near it, allowing it to act as both an acid and base during catalysis

C. Absorption would decrease significantly
At pH 4.0, ibuprofen is ~71% protonated (uncharged), but at pH 1.5 it was >99% protonated; this ~28% increase in charged form would reduce membrane permeability.
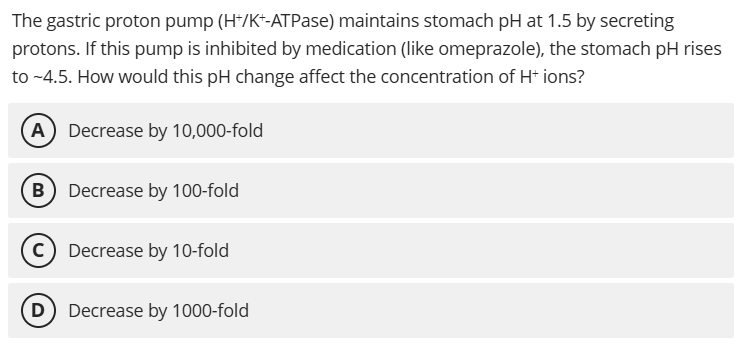
D. Decrease by 1000-fold
pH change from 1.5 to 4.5 is 3 units, and since pH = -log [H+], the H+ concentration decreases by 10³ = 1000-fold

D. Insulin is negatively charged and would become less negatively charged
At pH 7.0, insulin (pI 5.3) carries a negative charge; at pH 7.1 (still > pI) it remains negatively charged but slightly less so
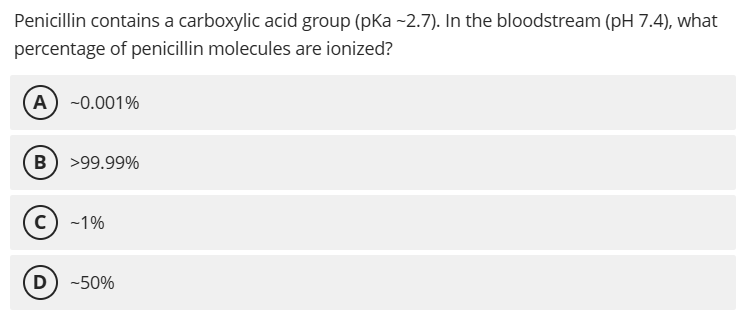
B. >99.99%
pH - pKa = 7.4 - 2.7 = 4.7, so [A⁻]/[HA] = 10^4.7 ≈ 50,000, meaning >99.99% is ionized; this high ionization prevents penicillin from entering human cells, contributing to its selective toxicity against extracellular bacterial infections.

C. Decreases by ~1.6 fold
at pH 7.4: ratio = 20; at pH 7.2: ratio =12.6; this represents a 1.6-fold decrease (20/12.6)