3.1.11 Electrode potentials and electrochemical cells
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
Zn(s) ⇌ Zn2+(aq) + 2e-
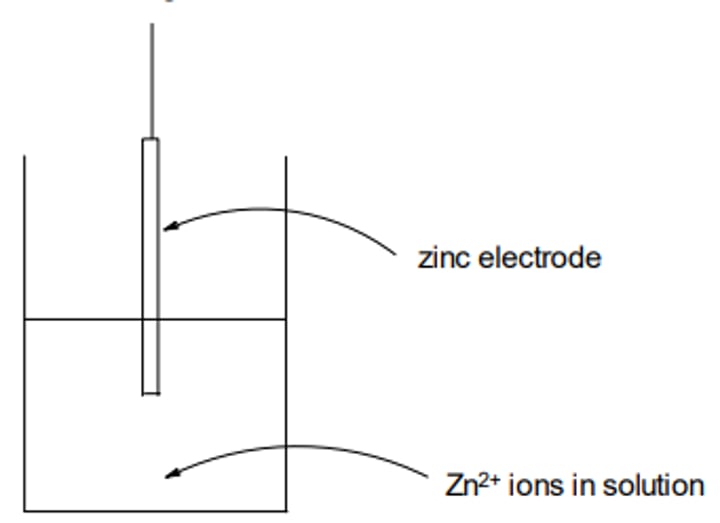
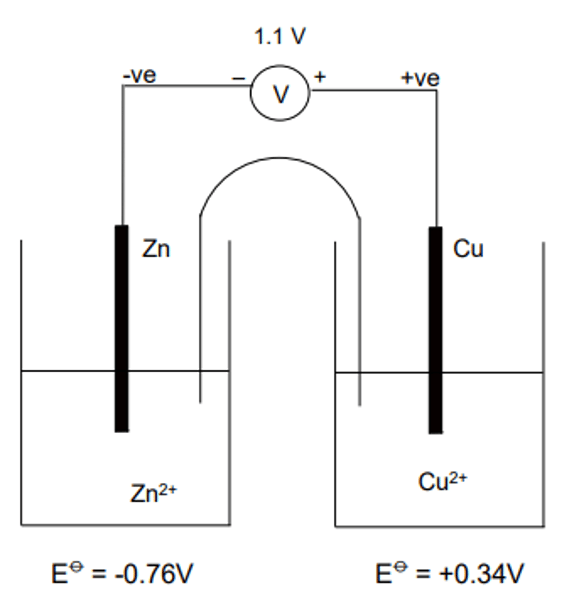
Electrochemical Cells
If two electrodes are connected using a metal wire through a high resistance voltmeter and the circuit completed using a salt bridge, then the potential difference between the two electrodes can be measured.
Example- Electrochemical Cells
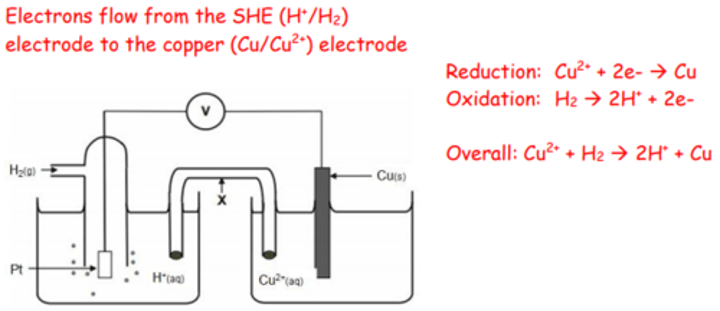
Electrons flow from the zinc electrode to the copper electrode.
Reduction takes place at the copper electrode
(RHS):
Cu2+(aq) + 2e—> Cu(s)
Oxidation takes place at the zinc electrode
(LHS):
Zn(s)—> Zn2+(aq) + 2e
The overall cell reaction is:
Zn(s) + Cu2+(aq)—> Zn2+(aq) + Cu(s)
Importance of the salt bridge
The salt bridge allows ions to flow and completes the circuit. It is often just a piece of filter paper that has been soaked in a salt solution.
Examples of salts that are usually used in a salt bridge:
KNO3, KCl
Identify the key features of choosing a salt for producing a salt bridge:
The salt should not contain any of the ions in either of the electrodes; this will stop the conditions from being standard and move the position of equilibrium.
The salt should not contain any ions that would react with any of the ions in the electrodes; e.g. a hydroxide would react with H+ ions.
Electrochemical Cells:
In an electrochemical cell the electrons flow from the oxidation electrode to the reduction electrode, this generates an electromotive force (emf) which is measure in volts (V).
EMF = Red - Ox
The two electrodes are connected by a voltmeter and a salt bridge; the salt bridge completes the circuit and allows ions to move and carry the charge. It is important that the salt used is inert so that it does not react with the electrodes and does not contain any of the ions in the electrodes to prevent a change to the cell conditions.
Standard Hydrogen Electrode:
The SHE is used to measure the electrode potentials (Eθ) of each half-cell. By convention the SHE is shown on the LHS of diagrams.
The SHE has an E value of 0.00V by definition, it contains 1.0 moldm-3 H+ ions from HCl, and the H2 gas is pumped into the cell at 100 kPa and 298K.
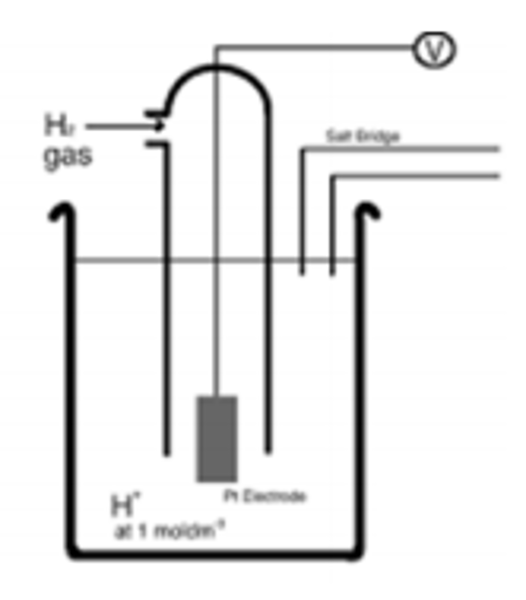
The standard hydrogen electrode is given a value of 0.00V
(BY DEFINITION)
The electrode equation is:
H+(aq) + e- ⇌ ½ H2 (g)
E⦵ = 0.00V
You need to be able to draw and label the
standard hydrogen electrode.
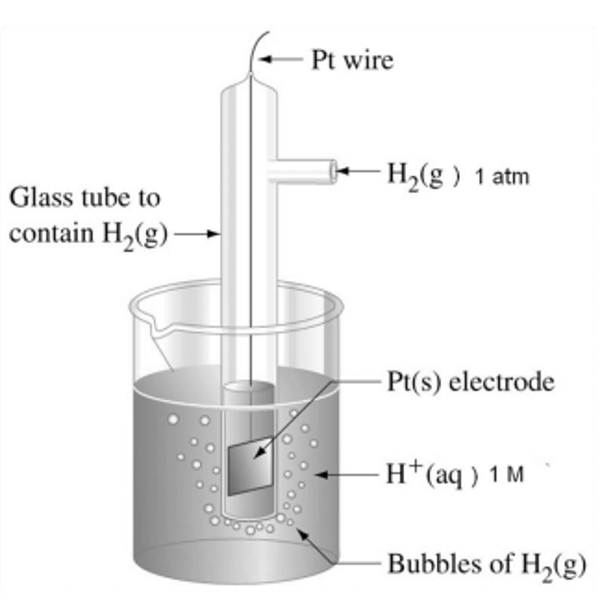
Standard Conditions:
Electrode potentials (Eθ) are measured using standard conditions:
All solutions: 1.0 moldm-3
Temperature: 298 K
Pressure of gases: 100 kPa
Measured in the absence of current
using a high-resistance voltmeter
Draw a diagram of a copper half-cell attached to a SHE. Use arrows to show the direction of the electrons. Write two half-equations and the overall cell equation.
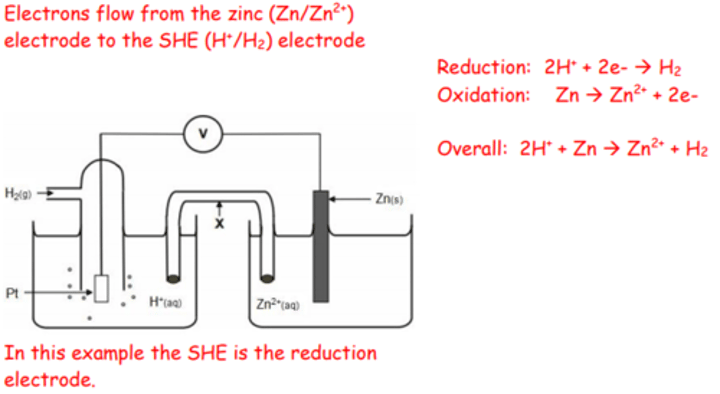
Draw a diagram of a zinc half-cell attached to a SHE. Use arrows to show the direction of the electrons. Write two half-equations and the overall cell equation.
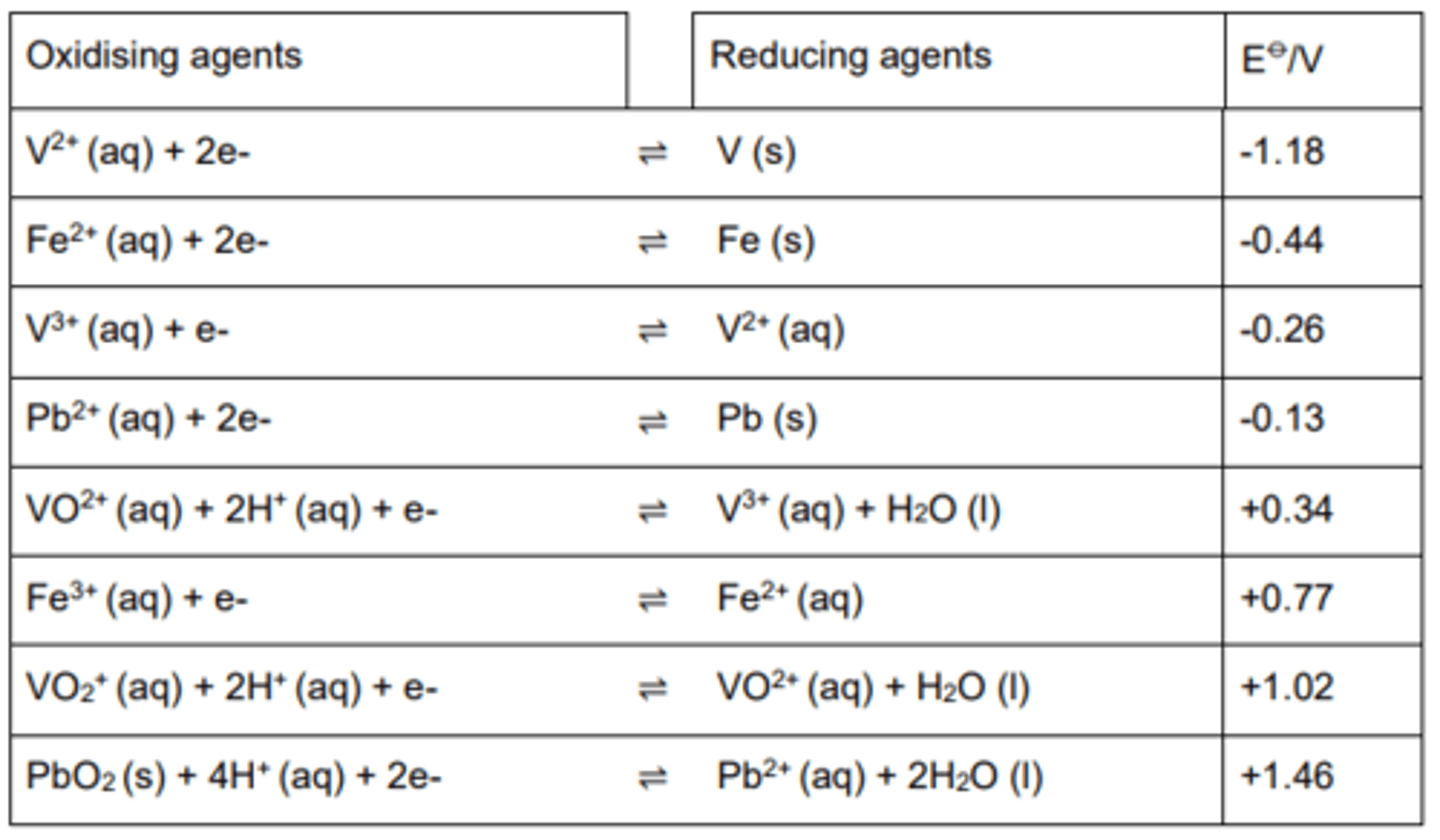
Electrochemical Series:
The electrochemical series lists all Eϴ
for half-cells (usually in order). All the equations are written as reduction.
In this set of data V2+ is the strongest reducing agent because it is the reactant in the reverse reaction with the least positive Eϴ.
While MnO4- is the strongest oxidising agent because it is the reactant in the forwards reaction with the most positive Eϴ.
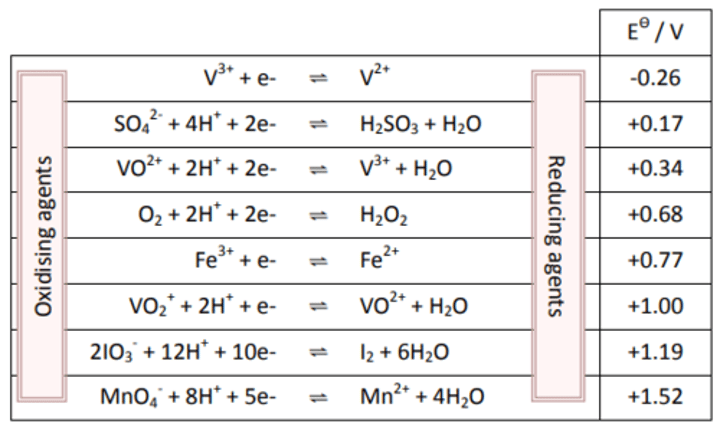
Note:
All half-equations in the table are written as reduction reactions.
The electrode with the most positive E⦵ value is the reduction process and the forwards reaction.
Electrodes with a more positive E⦵ value can oxidise an electrode with a less positive E⦵ value.
When combining the two half-cell reactions the half reaction with the least positive value is reversed.

Calculating the e.m.f. of a cell

Determining the overall equation:
The Zn electrode has the lower E⦵ so is reversed.
Zn ⇌ Zn2+ + 2e-
Cu2+ + 2e- ⇌ Cu .
Zn + Cu2+ ⇌ Zn2+ + Cu
Zn is the reducing agent: it supplies the electrons to reduce the Cu2+
Cu is the oxidising agent: it accepts electrons from the Zn.
Feasible Reactions:
For these reactions only one part of each electrode is given.
A reaction is feasible if the emf is positive, it is important to identify which of the two species is being oxidised and which is reduced.
● E.g. Is a reaction feasible between IO3- and V3+?
In the given set of data IO3- is an oxidising agent, so V3+ is potentially being oxidised:
emf = 1.19 - 0.34 = +0.85 V, positive so feasible.
● E.g. Is a reaction feasible between H2O2 and VO2+?
In the given set of data H2O2 is a reducing agent, so VO2+ is potentially being reduced:
emf = 0.34 - 0.68 = -0.34 V, negative so not feasible.
Further Products:
● What is the final oxidation product when V2+ is added to Fe3+?
Fe3+ is a medium strength oxidising agent, it will oxidise V2+ to V3+ (emf = +1.03V), it can also oxidise V3+ to VO2+ (emf = +0.43V), however it cannot
oxidise VO2+ to VO2+ (emf = -0.23V) so the final oxidation product is VO2+.
Predicting if a reaction is feasible
A reaction is feasible if the E⦵cell (emf) is zero or positive;
However these reactions may not occur if the activation energy is too high or the reaction will
not proceed under standard conditions.
For a reaction to be feasible the forward reaction must be the more positive reaction and
have a more positive E⦵ value.
1) Will lead displace aqueous copper (II) ions in solution?
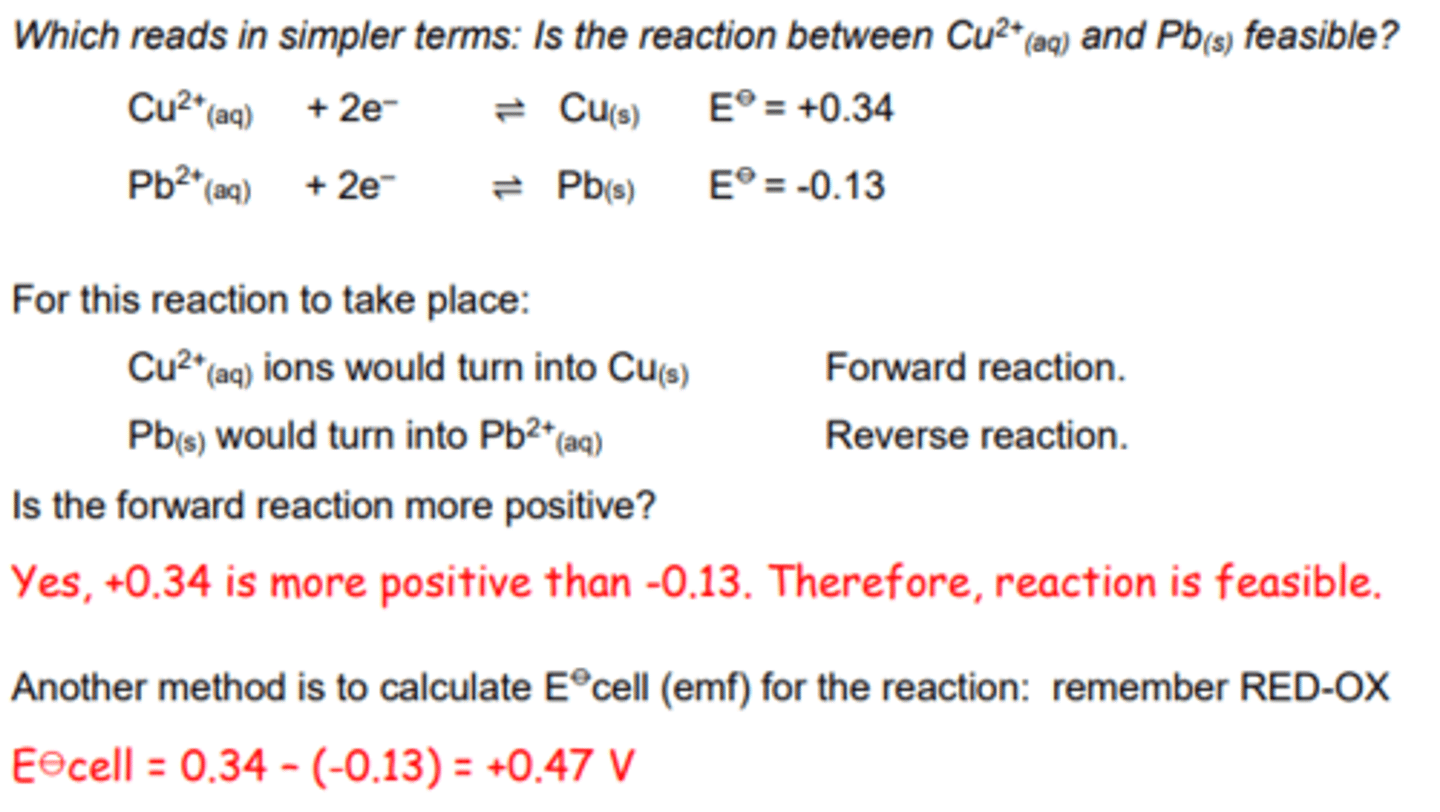
Identifying oxidising and reducing agents
Oxidising agents are the reactant in the forwards half-equation:
• The strength of an oxidising agent is determined by how readily it accepts electrons:
most positive E⦵
Reducing agents are the reactant in the backwards half-equation:
• The strength of a reducing agent is determined by how readily it donates electrons:
least positive E⦵
Predicting products of redox reactions
Vanadium metal (V(s)) can be oxidised to V2+(aq) ions, which can then be oxidised to V3+ (aq) ions, which can then be oxidised to: VO2+(aq) ions, which can then be oxidised to VO2+ (aq) ions.
However, complete oxidation can only be achieved using very strong oxidising agents.
With weaker oxidising agents then the V would only be oxidised to one of the other species.
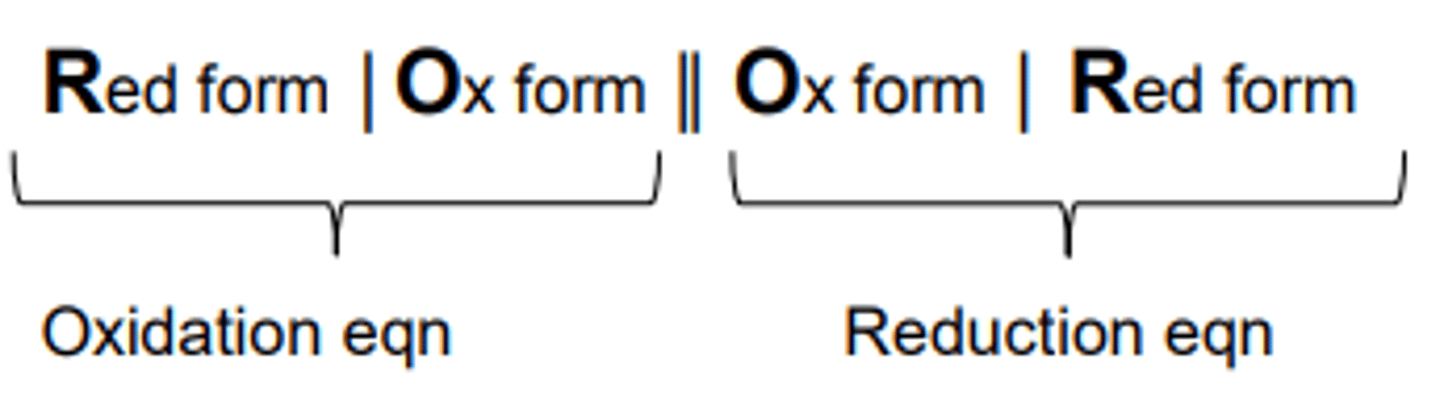
Cell Notation: Remember: ROOR
The reduction electrode is written on the RHS, phase boundaries are shown with a line, whilst the salt bridge is a double line in the middle.
Platinum electrodes are needed if there is no solid metal in the half-eqn.
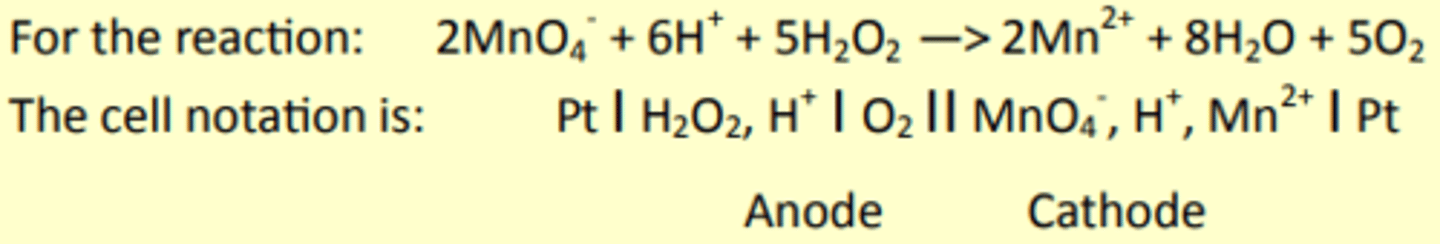
Cell notation
When writing a cell diagram the electrode at which Reduction occurs goes on the RHS.
The vertical single line represents any phase change or change of state, the double line is the salt bridge.
If two species are in the same phase then a comma is used to separate them.
If the electrode only involves aqueous solutions or a gas and aqueous solution then a platinum electrode must also be used to allow the electrons to move to and from the solution.
If a platinum electrode is being used it is places at either end of the cell notation diagram.
Water does not appear in the cell notation, it is referred to by using the (aq) state symbol.
H+ ions are not oxidised or reduced (unless in the SHE) and usually appears between the two redox forms in the cell notation.
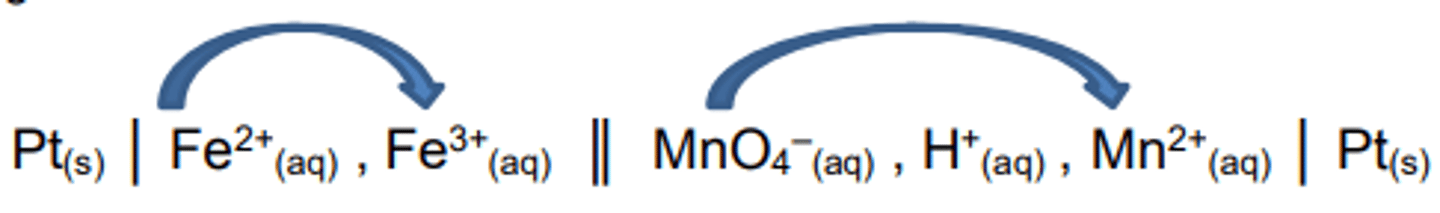
Writing the overall equation from the cell notation
When writing the overall equation of a reaction from the cell notation you read the information from left to right.
In the oxidation electrode (LHS) the Fe2+ reacts to form Fe3+.
In the reduction electrode (RHS) the MnO4-reacts to form Mn2+.
Changing cell conditions:
The half-cell reactions are reversible and so Le Châtelier's principle applies.
If a condition is changed and the position of equilibrium shifts to RHS then the E⦵cell increases. If the position of equilibrium shifts to LHS the E⦵cell decreases.
You should explain this as more/less oxidation or reduction occurring:
More reduction and more oxidation increases E⦵cell
Less reduction or less oxidation decreases E⦵cell.
1) If the [Cu2+] was increased predict what would happen to:
a. The position of equilibrium
b. The value of E⦵cell
a. move to RHS; more reduction would occur
b. increases
Changing Cell Conditions
Changing conditions to favour the forwards reaction increases the emf value because there will be either more oxidation or more reduction.
E.g. State and explain the affect to the emf of the cell if [Fe3+] increases.
Pt I H2SO3, H+, SO42-II Fe3+, Fe2+ I Pt
Reduction equation is Fe3+ + e- —> Fe2+, when [Fe3+] increases, the position of equilibrium in the overall equation moves to the right as there will be more reduction so the emf will increase.
Alternatively if the backwards reaction is favoured then there would be less oxidation or less reduction which would reduce the emf value of the cell.
Commercial Electrochemical Cells and Batteries
There are 3 types of commercial electrochemical cells:
1. Non-rechargeable batteries
2. Rechargeable batteries
3. Fuel cells
1. Non-rechargeable (irreversible)
These cells are not rechargeable because the reaction cannot be driven in the backwards direction, the reaction is NOT reversible.
Zinc/Copper - Daniell Cell
This was the first portable electrochemical
cell. However it is impractical to use because of the difficulty in transporting the liquids (zinc sulphate solution and copper sulphate solution).
It also produced very little power.

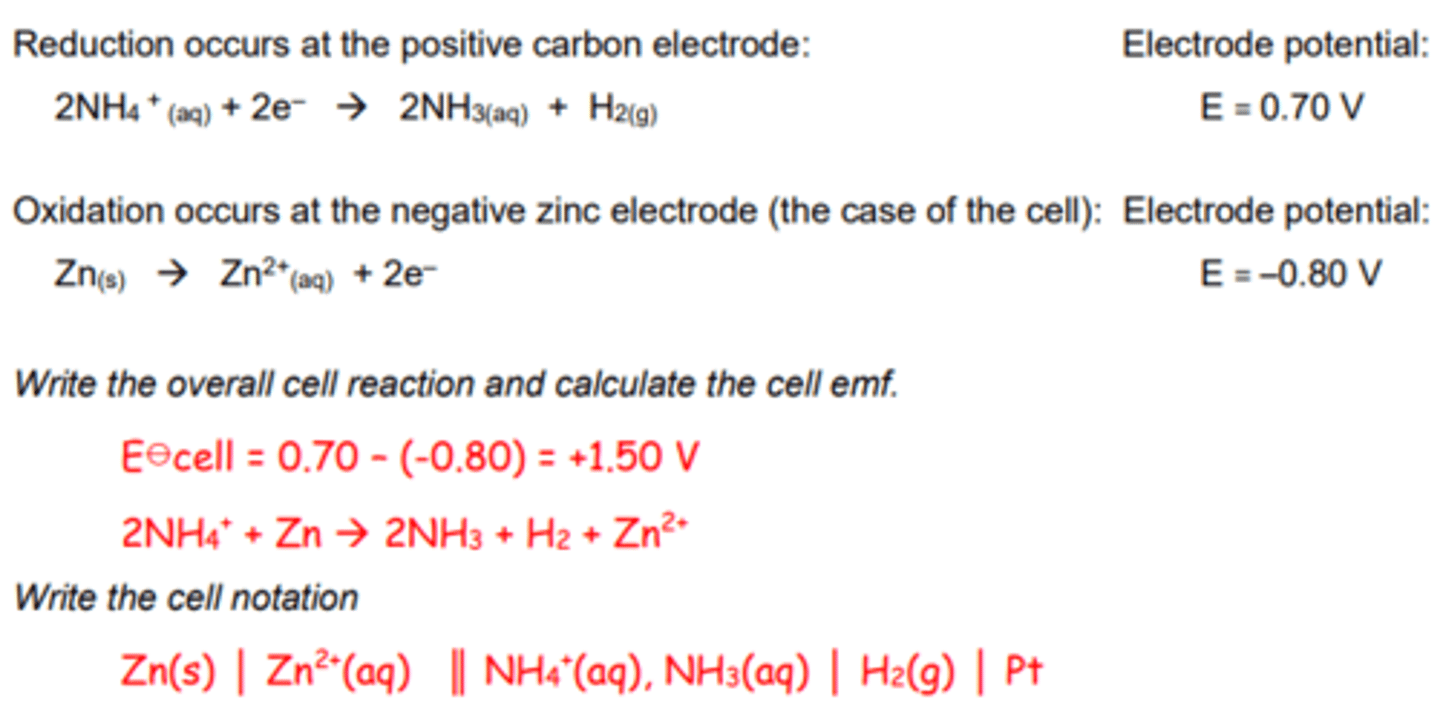
Zinc Carbon Cells
In the original form of this cell (Leclanché
cell) the electrolyte is an acidic paste of
ammonium chloride and water.
The hydrogen gas produced by the reaction is oxidised to water by the presence of MnO2 in the cell.
The ammonia dissolves in water in the paste
in the cell.
As the zinc is used up the cell walls become
thinner and the acidic solution can leak out.
This type of battery tends to be used in gadgets that only use a small amounts of power for short periods of time, e.g. TV remote, smoke alarm.
Advantages and disadvantages of non-rechargeable batteries
Advantages:
Small and cheap portable power source.
Disadvantages:
Waste and disposal issues (only used once and then thrown away).
New government initiative - major retailers to offer disposal/recycling.
2. Rechargeable batteries
These reactions are reversible:
When the battery is being used the overall reaction is proceeding in the forwards direction.
When the battery is being recharged then the overall reaction is proceeding the backwards direction; if you are asked to write the equation for recharging you MUST write the backwards reaction out.
Lead Acid
One electrode is lead and the other is lead, but coated with lead oxide.
At the Pb electrode:
Pb(s) + SO42-(aq) -> PbSO4(s) + 2e-
At the oxide coated electrode:
PbO2(s) + 4H+(aq) + SO42-(aq) + 2e-
-> PbSO4(s) + 2H2O(l)

b) Portable Batteries
i) Nickel cadmium
Positive electrode:
NiO(OH)(s) + H2O(l) + e-
-> Ni(OH)2(s) + OH-(aq)
E⦵= +0.52V
Negative electrode:
Cd(OH)2(s) + 2e- -> Cd(s)+ 2OH-(aq)
E⦵= -0.88V
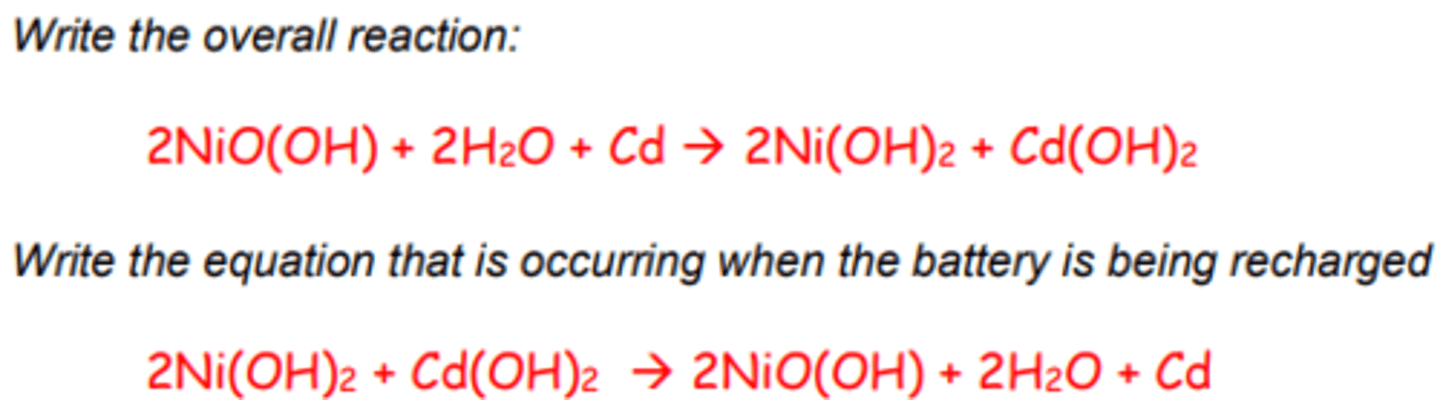
b) Portable Batteries
ii) Lithium ion
You need to know the simplified half-equations
given here:
Positive electrode:
Li+ + CoO2 + e- -> Li+[CoO2]-
Negative electrode:
Li -> Li+ + e-
In a lithium-ion battery the lithium ions move from the negative electrode to the positive electrode during discharge and back when charging.
![<p>You need to know the simplified half-equations<br>given here:<br>Positive electrode:<br>Li+ + CoO2 + e- -> Li+[CoO2]-<br>Negative electrode:<br>Li -> Li+ + e-<br>In a lithium-ion battery the lithium ions move from the negative electrode to the positive electrode during discharge and back when charging.</p>](https://knowt-user-attachments.s3.amazonaws.com/6bdcb390-1764-4a76-a483-46f3d8fc3bb5.png)
Advantages and disadvantages of rechargeable batteries
Advantages:
There is less waste as they are reused, this often works out cheaper over time.
Usually provide more power than non rechargeable batteries.
Disadvantages:
There are still disposal issues, when they can no longer be recharged.
Lithium ion batteries should be recycled to conserve resources.
3. Fuel Cells - an alternative to burning the hydrogen
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction.
Made from two platinum electrodes with a
polymer electrolyte - allows passage of ions.
These cells do not need to be recharged but
are refuelled like a normal petrol/diesel
engine
i) Acidic conditions:
(Anode) Negative electrode:
H2(g) -> 2H+(aq) + 2e-
E = 0.0 V
(Cathode) Positive electrode:
O2(g) + 4H+(aq) + 4e- -> 2H2O(l)
E = 1.23 V

ii) Alkaline conditions
The fuel cell can also operate under alkaline conditions.
(Anode) Negative electrode:
H2(g) + 2OH- -> 2H2O (l) + 2e-
E = -0.83 V
(Cathode) Positive electrode:
O2(g) + 2H2O (aq) + 4e- -> 4OH-(l)
E = 0.40 V

Advantages and disadvantages of fuel cells
Advantages:
Doesn't need to be recharged, only refuelled.
Only product is water: No pollution.
Disadvantages:
Very expensive.
Production of hydrogen; either:
through electrolysis (high electricity use and costs) or
produced from methane (a non-renewable resource)
CH4(g) + H2O(l) 3H2 (g) + CO(g)
HSW Commercial Cells:
Re-chargeable batteries:
Re-chargeable batteries: are small, portable and have reduced disposal issues. They can be recharged because the reverse reaction occurs when they are connected to an outside electrical source.
E,g, Lithium ion batteries
Reaction when using:
Li + CoO2 —> Li+[CoO2]-
Reaction when recharging:
Li+[CoO2]- —> Li + CoO2
HSW Commercial Cells:
Fuel cells:
Fuel cells: convert chemical energy from a fuel into electricity, the hydrogen-oxygen fuel cell is greener because only H2O is produced, however the H2 is currently sourced from fossil fuels.
The fuel cell reactions can occur under acid or alkaline conditions, the same voltage is produced because the overall equations are the same.