VSEPR Theory Chart
1/14
Earn XP
Description and Tags
Essentially electron configurations
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms

Arrangement?
Linear (180deg, sp oribital, no dipole)
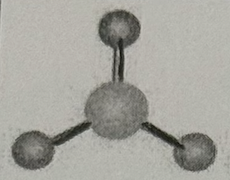
Arrangement?
Trigonal Planar (120deg, sp² oribital, no dipole)
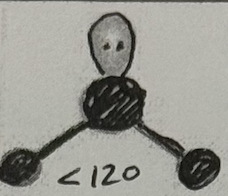
Molecular Geometry?
Bent (<120deg, sp² oribital, yes dipole)
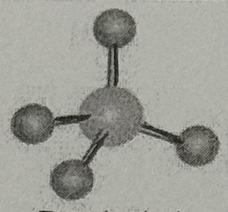
Arrangement?
Tetrahedral (109.5deg, sp³ oribital, yes dipole)
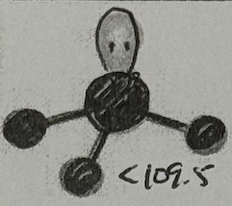
Molecular Geometry?
Trigonal Pyramidal (<109.5deg, sp³ oribital, yes dipole)
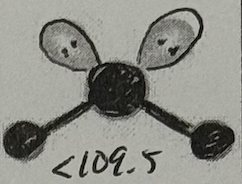
Molecular Geometry?
Bent (<109.5deg, sp³ oribital, yes dipole)
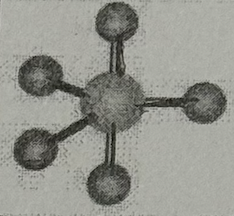
Arrangement?
Trigonal Bipyramidal (120+90deg, sp³d oribital, no dipole)
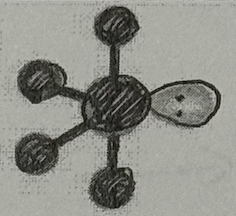
Molecular Geometry?
Seesaw (<120+90deg, sp³d oribital, yes dipole)
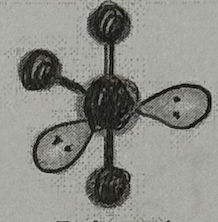
Molecular Geometry?
T-Shaped (<120+90deg, sp³d oribital, yes dipole)
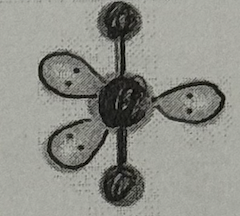
Molecular Geometry?
Linear (<120+90deg, sp³d oribital, no dipole)
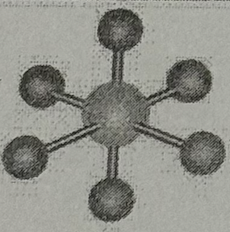
Arrangment?
Octahedral (90deg, sp³d² oribital, no dipole)
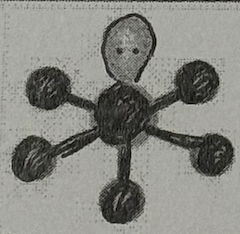
Molecular Geometry?
Square Pyramidal (<90deg, sp³d² oribital, yes dipole)
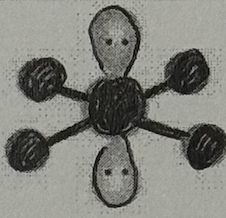
Molecular Geometry?
Square Planar (<90deg, sp³d² oribital, no dipole)
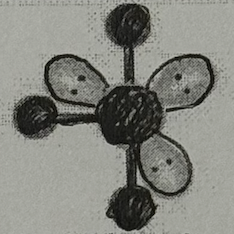
Molecular Geometry?
T-shaped (<90deg, sp³d² oribital, yes dipole)
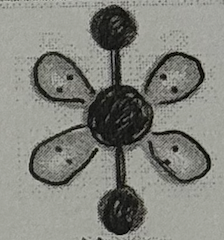
Molecular Geometry?
Linear (<90deg, sp³d² oribital, no dipole)