10 - Organic Chemistry
1/23
Earn XP
Description and Tags
10.1 - Basics 10.2 - Functional Group Chemistry 20.1 - Organic Reactions 20.2 - Synthetic Routes 20.3 - Stereomerism
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
Organic Chemistry
focuses on compounds containing carbons, biological molecules like simple sugars to nucleic acids, fossil fuels like coal and natural gas, synthetic materials like nylon and Lycra, domestic and industrial products like paints and detergents.
Organic Compound
a compound containing carbon, most of the time also hydrogen in a covalent bond. Oxygen, nitrogen, chlorine, sulfur are often present as well.
Why is carbon important?
Carbon forms four strong covalent bonds with other elements, namely hydrogen. Carbon is also capable of catenation, link to itself forming chains or rings.
Homologous series
families of compounds with the same functional group, similar chemical and physical properties that can be represented by the same general formula. For instance, Alkanes are represented by the general formula CnH2n+2, and each successive member increases by a fixed amount of molecular mass.
Homologous series and Physical properties
As successive members of a homologous series differs by CH2 groups they possess longer carbon chains and exhibit gradual trends in their physical properties.
Boiling point increases as the molecular weight and size increase, hence induced dipoles cause stronger London dispersion forces between molecules, requiring more energy for transition between liquid and gas. The increase is steeper initially as the increased chain length is relatively greater for small molecules. CH4 is a gas at room temp and C8H18 is a liquid,
Density increases as molecular weight increases, hence more mass per unit volume.
Viscocity increases as molecular weight increases and larger molecules experience more resistance to flow due to more complex structure.
Homologous series and Chemical properties
Homologous series show trends in chemical properties as they have the same functional group. If the traits of a functional group are know the properties can be predicted for all members of a series.
Reactivity: Alcohols with -OH functional group can be oxidized to form organic acids, the -COOH functional group in Carboxylic acids is responsible for acidic properties
Functional group
an atom or group of atoms attached to a carbon atom in a molecule that provides a compound with certain chemical properties, denoted by R - rest of the molecule
Organics and Empirical formulae
The empirical formula shows the simplest whole number ratio of the atoms it contains, it can be derived from percentage composition data.
Organics and Molecular formulae (P 467)
The molecular formula shows the actual number of atoms of each element, if the molecular formula is a multiple of the empirical formula and Molar mass, M, the relationship M = (molar mass of empirical formula)n can be applied.
Structural formula
a representation of the molecule showing how atoms are bonded to each other, show bonds and bond angles.
Stem identifies the number of carbon atoms in longest chain:
Meth- = 1C, Eth- = 2C, Prop- = 3C, But- = 4C, Pent- = 5C, Hex- = 6C, e.g: Methane = CH4, Hexane = C6H14.
Mnemonic: Methhead Ethel Propels Butterfly Pentagram Hexes
Suffix endings and Nomenclature
suffix identifies, class, functional group and general formula
Suffix | Class | Functional Group | General Formula |
|---|---|---|---|
ane | alkane | no functional group | CnH2n+2 |
ene | alkene | alkenyl | CnH2n |
yne | alkyne | alkynyl | CnH2n-2 |
anol | alcohol | hydroxyl | CnH2n+1OH |
oxylakane | ether | ether | R - O - R’ |
anal | aldehyde | aldehyde/carbonyl | R - CHO |
anone | ketone | carbonyl | R - CO - R’ |
anoic acid | carboxylic acid | carboxyl | CnH2n+1COOH |
anoate | ester | ester | R - COO - R’ |
anamide | amide | carboxyamide | |
anamine | amine | anamine | |
anenitrile | nitrile | anenitrile | |
benzene | arene | phenyl |
Position of a Functional group
The position of a functional group is shown by a number between dashes inserted before the functional group ending, the number refers to the carbon atom to which the functional group is attached, chain is numbered starting at the end that will give the smallest number to the group
Alcohol functional group
Alcohol functional group involves an oxygen atom bonded to a hydrogen atom, -OH, and a carbon atom which is part of a larger organic. R-OH.
Alkyl
An alkyl grou[ is formed by remove one hydrogen atom from an alkane chain, and so stem changes from -ane to -yl. The letter R is used to discuss an unspecific alkyl group.
E.g.: Methane CH4 - H = Methyl CH3
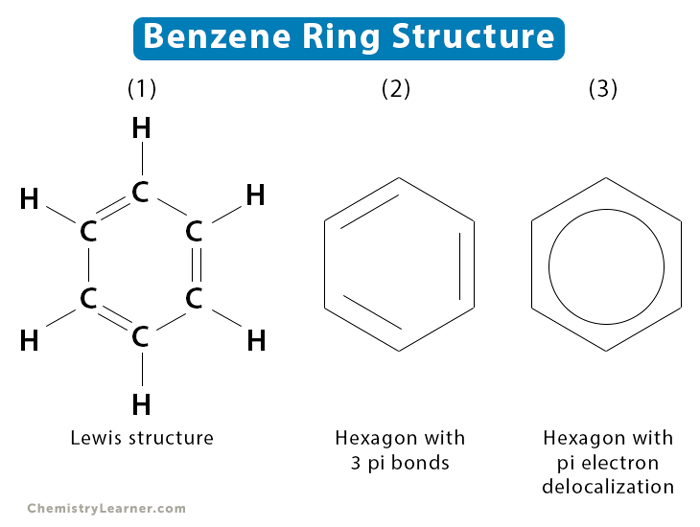
Aromatic compound
Aromatic compounds contain a benzene ring, C6H6,
Saturated Reactant
Compounds containing only single bonds, like alkanes
Unsaturated reactant
Compounds containing double or triple bonds, like alkenes, arenes
Aliphatic reactant
Aliphatics are saturated or unsaturated compounds without a benzene ring, like alkanes or alkenes
Arene reactant
Arenes are unsaturated compounds containing a benzene ring, like benzene or phenol
Electrophile
A species missing an electron hence attracted to parts of molecules that are electron-rich, electrophiles are positive ions or have partial + charges, like H+
Nucleophile
An electron-rich species hence attracted to parts of molecules which are missing electrons, nucleophiles have a lone pair of electrons and may have negative charges, like Cl-.
20.2 START
Nucleophilic Substitution
In nucleophilic substitution reactions a nucleophile species with a lone electron pair replaces the functional group of within an electrophile molecule.