Chemical reactions and equation- chp 1
Chemical reaction: %%It is a process where one or more substances foamed new substances%% and it can’t be reversed by physical methods
changes that can occur in a chemical reaction are:
- change in state
- change in color
- change in temperature
- evolution of gas
chemical changes happen in our daily life like
- digestion of food
- respiration
- rusting of iron
- formation of curd
Chemical equation
Chemical reactions are written in reactants and products like
magnesium + oxygen → magnesium oxide
However, instead of using words, %%we use%% %%symbols%% %%to represent reactants and products with the mention of their physical state.%%
Mg(s) + O₂(g) → MgO(s)
there are four physical state
- (s) -solid
- (l) -liquid
- (g)- gas
- (aq)- aqueous: it is a solution in which the solvent is water.
How to balance a chemical equation?
Law of conversion of mass: %%Mass can neither be created nor destroyed in a chemical equation.%%
That means if we weigh the reactants before the reaction and after the reaction, their weight will remain the same or the number of atoms of each element remain the same, before and after a chemical reaction.
%%we need to balance chemical reactions to have the same number of atoms in the reactant and the product.%%
The way we can balance the equation is:
Let’s take Fe + H₂O → Fe₃O₄ + H₂
STEP 1: to balance an equation, first draw a box (we will be underlining here) around the formula.
Fe + H₂O → Fe₃O₄ + H₂
STEP 2: list the atom of different elements in the equation
| Elements | number of atoms in the reactant (LHS) | number of atoms in the product (RHS) |
|---|---|---|
| Fe | 1 | 3 |
| H | 2 | 2 |
| O | 1 | 4 |
STEP 3: Start balancing the equation. The one with the most number of atoms. So here we will take Oxygen first.
| Elements | number of atoms in the reactant (LHS) | number of atoms in the product (RHS) |
|---|---|---|
| Fe | 1 | 3 |
| H | 2(4)= 8 | 2 |
| O | 1(4) = 4 | 4 |
Fe + 4 H₂O → Fe₃O₄ + H₂
Oxygen is balanced however hydrogen has become unbalanced so next, we will balance hydrogen.
(remember that we cannot alter the formula of the compounds or the element involved in the reaction.)
STEP 4: Now we will balance H atoms.
| Elements | number of atoms in the reactant (LHS) | number of atoms in the product (RHS) |
|---|---|---|
| Fe | 1 | 3 |
| H | 2(4)= 8 | 2(4)= 8 |
| O | 1(4) = 4 | 4 |
Fe + 4 H₂O → Fe₃O₄ + 4 H₂
STEP 5: balancing Fe atoms.
| Elements | number of atoms in the reactant (LHS) | number of atoms in the product (RHS) |
|---|---|---|
| Fe | 1(3)=3 | 3 |
| H | 2(4)= 8 | 2(4)= 8 |
| O | 1(4) = 4 | 4 |
3 Fe + 4 H₂O → Fe₃O₄ + 4 H₂
STEP 6: now recheck the equation to see if everything is balanced
3 Fe + 4 H₂O → Fe₃O₄ + 4 H₂
| Elements | number of atoms in the reactant (LHS) | number of atoms in the product (RHS) |
|---|---|---|
| Fe | 3 | 3 |
| H | 8 | 8 |
| O | 4 | 4 |
STEP 7: good job! however, there is one more thing needed to be done. Writing their physical state.
3 Fe(s) + 4 H₂O(g) → Fe₃O₄(s) + 4 H₂(l)
AND BALANCING IS DONE!

Types of chemical reactions
- combination reaction
- decomposition reaction
- displacement reaction
- double displacement reaction
- oxidation and reduction
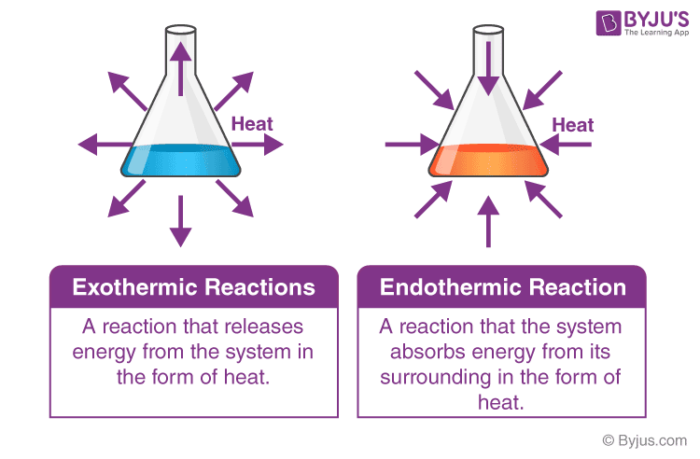
Before that let’s know in simple words about exothermic and endothermic reaction
Exothermic reactions: They are chemical reactions that produce heat.
Endothermic reaction: Chemical reactions in which the reactants absorb heat energy from the surroundings to form products.
combination reaction: %%Two or more compounds combine to form one compound.%%
general formula: A + B → AB
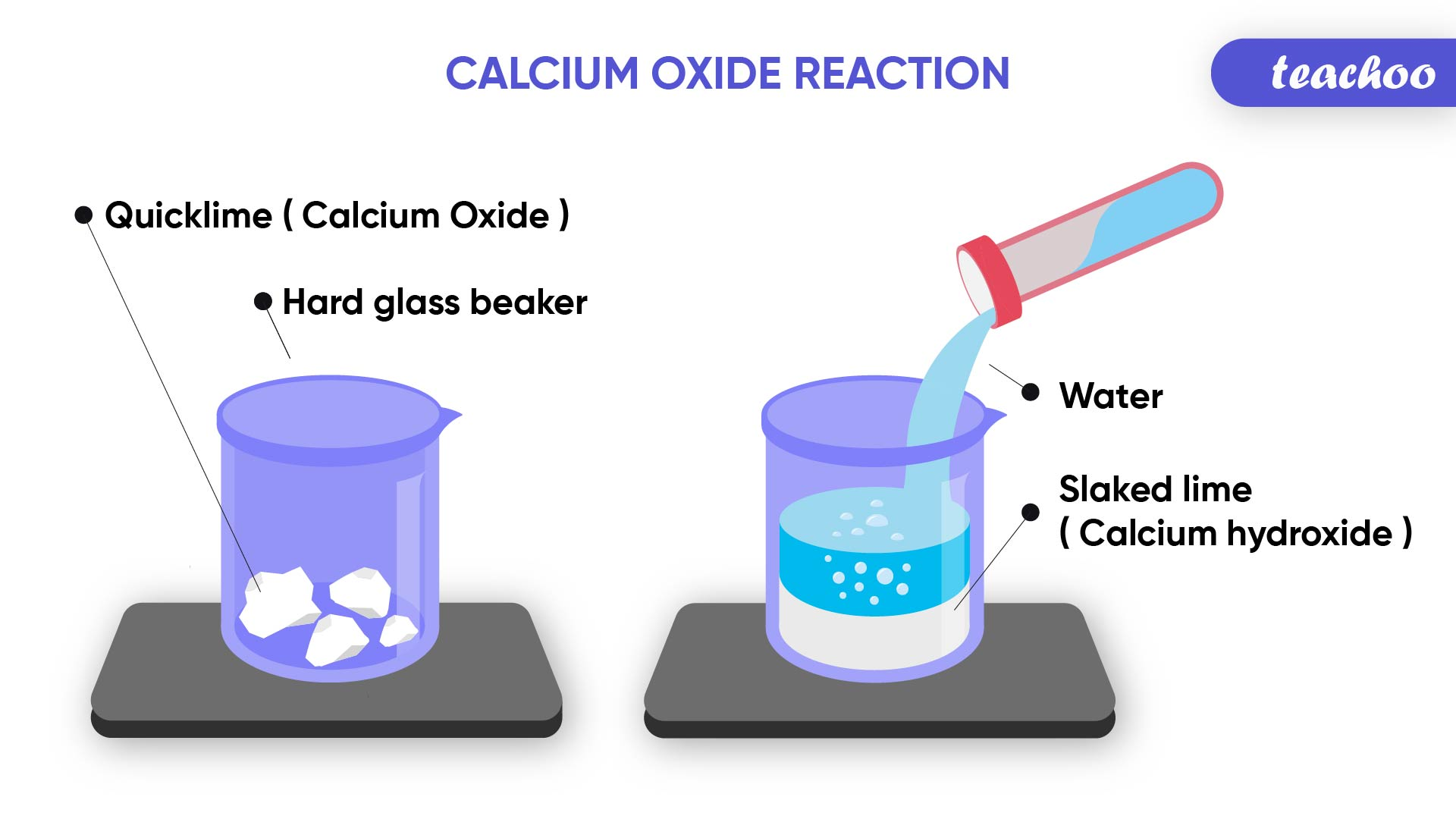
let’s do a small activity
- take a small amount of CaO or quick lime to a beaker
- slowly add water
- touch the beaker at the bottom
- there will be a change in temperature, here it has become hotter
calcium oxide reacts vigorously with water to produce larger amounts of heat. This is an exothermic reaction.
CaO(s) + H₂O(l) → Ca(OH)(aq) + heat
Did you know?
%%The solution of slaked lime produced by the above reaction is used for whitewashing walls.%% Calcium hydroxide reacts slowly with the carbon dioxide in the air to form a thin layer of calcium carbonate on the walls. Calcium carbonate is formed after two to three days of whitewashing and gives a shiny finish to the walls. Interestingly the chemical formula of marble is CaCO₃.
Ca(OH)(aq) + CO₂(g) → CaCO₃(s) + H₂O(l)

Other examples are
i) Burning of coal
C(s) + O₂(g) → CO₂(g)
ii) formation of water
2H₂(g) + O₂(g) → 2H₂O(l)
Decomposition reaction: The opposite of a combination reaction – a complex molecule breaks down to make simpler ones.
They often require heat, electricity, or light for breaking down the reactants. This type of reaction is also called endothermic reaction.
General formula: AB → A+B
let’s do a small activity
take about 2g of ferrous sulphate crystals in a dry boiling tube.
note the color of the ferrous sulphate crystals
heat the boiling tube over the flame of a burner or spirit lamp
observe the color of the crystals after heating
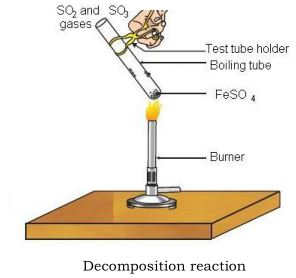 2FeSO₄(s)→∆Fe₂O₃(s) + SO₂(g)+SO₃(g)
2FeSO₄(s)→∆Fe₂O₃(s) + SO₂(g)+SO₃(g)ferrous sulphate crystals (FeSO₄, 7H₂O) lose water when heated, and the color of sulphate crystals changes. Then it decomposes to ferric oxide ( Fe₂O₃), sulphur dioxide (SO₂), and sulphur trioxide (SO₃).
CaCO₃(s) →∆ CaO(s) + CO₂(g)
- This reaction is used in many industries.
- This has many uses such as the manufacture of cement.
When a decomposition reaction is carried out by heating, it is called thermal decomposition.
An example of this is
2Pb(NO₃)₂(s) →∆ 2PbO(s) + 4NO₂(g) + O₂(g)
The emission of brown fumes is nitrogen dioxide (NO₂)
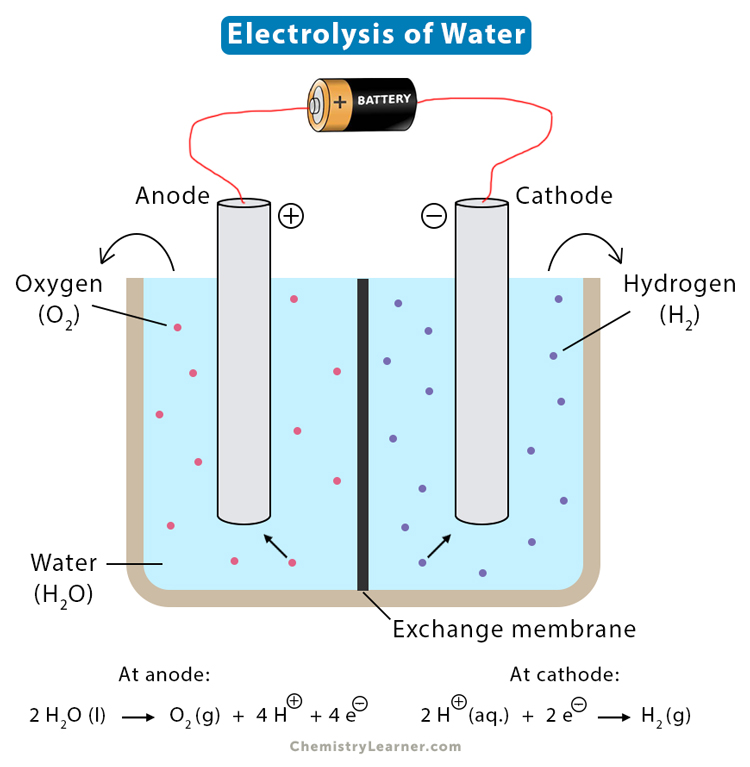
Electrolysis
The process of decomposing ionic substances into simpler substances when an electric current is passed through them is known as electrolysis.
Electrolysis of water
%%The chemical reaction of passing an electric current through water to decompose hydrogen and oxygen gases is known as the electrolysis of water.%%
If we place some silver chloride on a china dish and left in the sunlight for a while, what would be observed?
We will observe a change in color. It will turn from white silver chloride to grey.
2AgCl(s) → hv 2Ag(s) + Cl₂(g)
silver bromide also behaves similarly
2AgBr(s) → hv 2Ag(s) + Br₂(g)
the above reactions are used in black and white photography.
Displacement Reaction: One element takes place with another element in the compound.
general formula: A + BC → AC + B
Let's do a small activity
Take two test tubes marked (A) and (B). In each test tube, take about 10 ml of copper sulphate solution.
Tie two iron nails with a thread and immerse them carefully in the copper sulphate solution in test tube B for about 20 minutes. Keep one iron nail aside for comparison.
After 20 minutes, take out the iron nails from the copper sulphate solution.
Compare the intensity of the blue color of copper sulphate solutions in test tubes (A) and (B),
Also, compare the color of the iron nails dipped in the copper sulphate solution with the one kept aside.
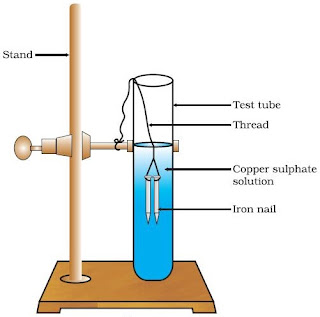
Fe(s) + CuSO₄ ⟶ FeSO4₄ + Cu(s)
The iron nail becomes brownish in color and the blue color of copper sulfate solution fades because iron has displaced another element, in this case, copper. from copper sulphate solution.
Other examples are
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
Pb(s) + CuCl₂(aq) → PbCl₂(aq) + Cu(s)
Zinc and lead are more reactive elements than copper. They displace copper from its compounds.
Double displacement reaction: A chemical reaction in which ions get exchanged between two reactants which form a new compound.
General formula: AB + CD → AC + BD
Let’s do a small activity
Take about 3ml of sodium sulphate solution in a test tube.
in another test tube, take about 3ml of barium chloride solution.
mix the two solutions together.
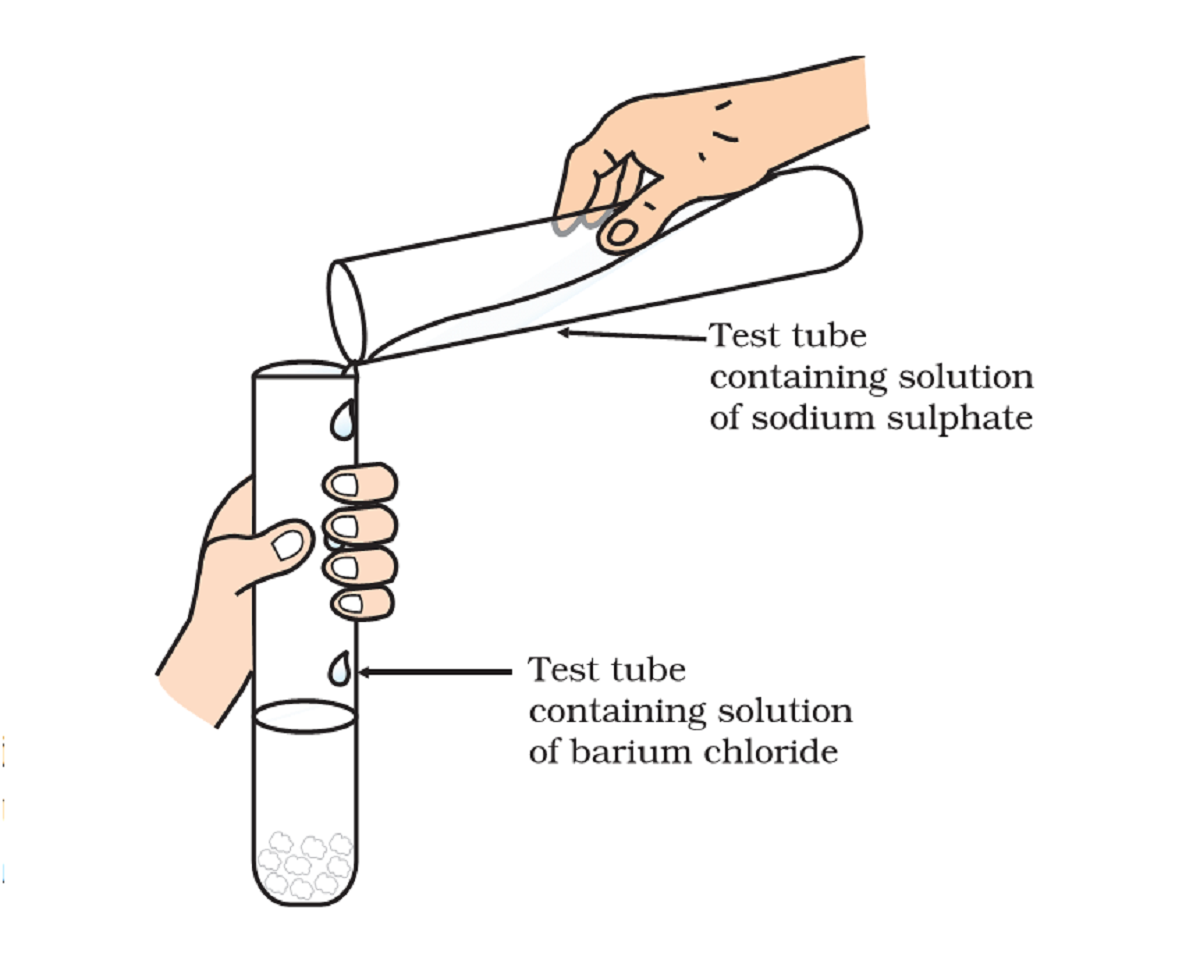
Na₂SO₄(aq) + BaCl₂(aq) → BaSO₄(s) + 2NaCl(aq)
You will observe that a white substance, which is insoluble in water, is formed. This insoluble substance formed is known as a precipitate.
Any reaction that produces a precipitate can be called a precipitation reaction.
The white precipitate of BaSO₄ is formed by the reaction of SO₄²⁻ and Ba²⁺. The other product formed is sodium chloride which remains in the solution.
Oxidation and Reduction reaction: A reaction involving both oxidation and reduction simultaneously means one reactant gets oxidized, and the other gets reduced.
This type of reaction is also called redox reaction.
if a substance gains oxygen or loses hydrogen it is oxidization.
if a substance loses or gains hydrogen it is reduction.
General formula: A+B → A⁺+B⁻
Let’s do a small activity
Put 1g copper power in a china dish.
Then heat it.
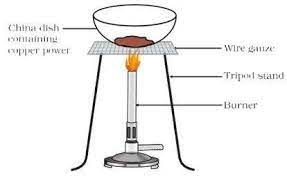
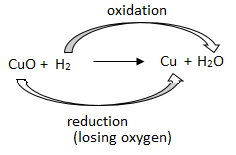
2Cu + O₂ →△ 2CuO
CuO + H₂ →△ Cu + H₂O
The surface of copper powder becomes coated with black copper(II) oxide because oxygen is added to copper, forming copper oxide.
If hydrogen gas is passed over this heated material (CuO), the black coating on the surface turns brown as the reverse reaction takes place and copper is obtained.
some other examples are:
ZnO + C → Zn + CO
MnO₂ + 4HCl → MnCl₂ + 2H₂O + Cl₂
The effects of Oxidation reaction in everyday life
Corrosion: Corrosion is the process of slowly eating up metals by gas and water vapours present in the atmosphere due to the formation of certain compounds like oxide, sulphides, carbonate, etc.
Example: Rusting of iron, Black coating on silver, and green coating on copper
Rancidity: When fats and oils stay in open for a long, they get oxidized and become rancid (old and stale), and their smell and taste change.
Prevention
- Adding antioxidants to food/….
- Keeping food refrigerator
- Storing it in an airtight container
- Keeping food away from light
- packaging food and oil in nitrogen gas, eg: chips