Bio Lab Midterm - Permeability
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
Diffusion
movement of molecules from a region of high concentration to a region of low concentration (move down the gradient)
Passive movement
diffusion of molecules or ions through a selectively permeable membrane (move down the gradient)
Dialysis
passive movement where one solute is separated from another solute due to a selectively permeable membrane allowing one solute to pass but not another
Osmosis
special case of passive movement in which solvent molecules (water) whose movement through the membrane is of interest.
hypotonic vs hypertonic
hypotonic - low solute concentration
hypertonic - high solute concentration
how does water move in comparison to hyper and hypotonic solutions
water moves from hypotonic solutions to hypertonic solutions (down water gradient)
solute
minor component in solution that is dissolved in solvent
solvent
major component in solution that dissolves solute (usually water)
solution
mixture where solute is dissolved in solvent
what is agar solution
agar is a polysaccharides. (it is heated then cooled to be gel like)
1% agar and 99% water
agar plate procedure
agar plate has two holes 1.5cm apart and when blue dye and HCl are put in both you will observe them diffuse through agar.
why use the dye
it is a pH indicator that changes from blue to yellow when pH drops below 4.0. We observed that there was yellow meaning H+ ions from HCl diffused towards the dye
molecular weight effect on diffusion
higher molecular weight results in a slower diffusion rate
Was the yellow closer to where the HCl started or the bromophenol blue dye?
It was closer to the bromophenol blue because HCl is lighter and diffuses faster.
Based on what you've seen here, do you think diffusion is an effective way to move substances from place to place in multicellular organisms? For example, would diffusion serve for moving inhaled oxygen from your lungs to your brain? Why/why not?
Diffusion is not an effective way to move substances from place to place in multicellular organisms because it is effective over short distances but due to the long time it takes to occur it would not be plausible.
dialysis tubing
membrane with tiny pores that we cannot see
Cut off value and what can pass through
The size biggest size of molecules that can pass through. In this lab it’s 12,000 Daltons so proteins and polysaccharides cannot pass through but carbohydrates and amino acids can.
Water gradient
Water concentration between two areas
osmotic pressure
Pressure that would have to applied to solution in the bag to prevent water on other side of membrane from diffusing into bag
How does osmotic pressure increase
Osmotic pressure increases with solute concentration
water has O.P. value of 0
solution with 10% salt has greater O.P. than solution with 5% salt
Colligative Property
properties of solution that depend on ratio of solute particles to solvent particles. Osmotic pressure depends on this
Colligative property with molecules that dissociate in water
0.1M glucose and 0.1M sucrose have the same O.P. but 0.1M NaCl has a higher O.P. because it has 0.1 Na+ and 0.1 Cl-
rate of diffusion/osmosis
how fast water diffuses into bag
Does osmosis happen indefinitely?
rate of osmosis would eventually level off due to reverse pressure from bag or the bag would burst
Measure the weight of bags with what?
Balance not a scale
tare weight
Weight of paper that we do not want to account for.
raw data and how to adjust
Data in tables that accounts for membrane weight, tie weight, and solution weight. We only want solution weight so subtract 0.4g for the membrane and tie.
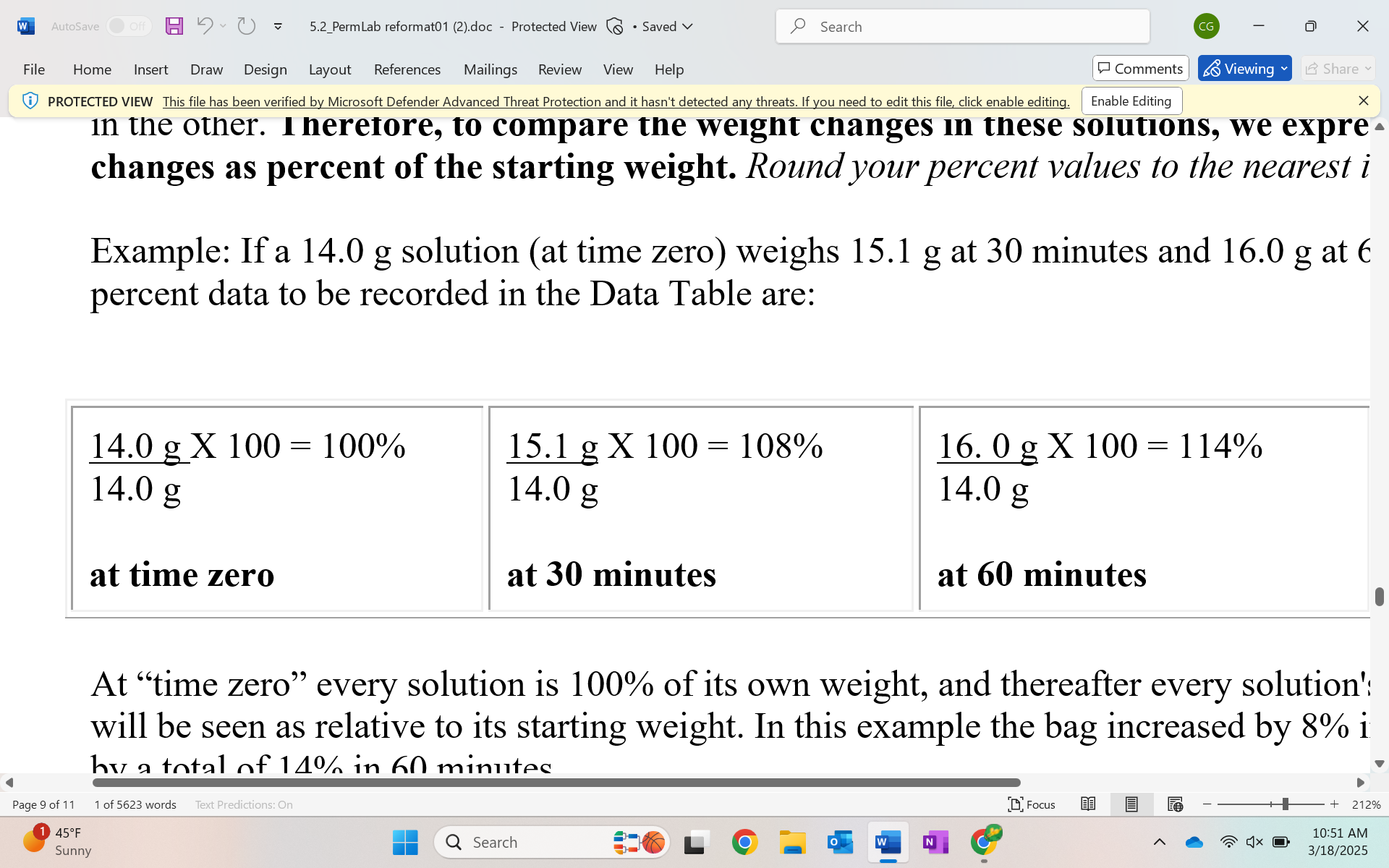
How do we express the weight change of solutions?
express them as a percentage of the starting weight.
When making the table what is the control and variable?
Control: Bag #1
Variable: Solute concentration or molecular weight
There should be no significant change in the weight of bag #1. Why? If you do find slight changes with time, how do you explain that?
There is no weight change because there is no water gradient as the concentration of water in the bag and out is the same. If any changes did occur it would be do to error as contaminants could have entered the water.
The bags with sucrose still gained weight even though both sucrose and water can diffuse freely, what does this tell us?
Water diffuses faster than sucrose as more water entered the bag than sucrose left meaning that water is smaller lighter than sucrose.