metals and reactivity
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
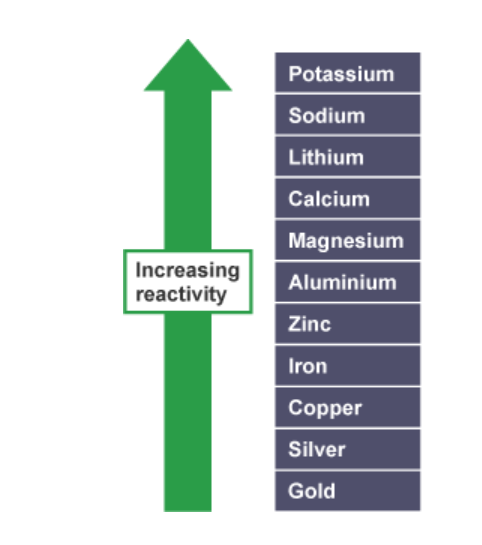
People Say Little Children Make A Zebra Ill Constantly Sniffing Giraffes.
metal + oxygen →
metal oxide
metal + acid →
salt + hydrogen
metal + water→
metal hydroxide + hydrogen
displacement reactions
a more reactive metal displaces a less reactive metal from a compound
what is a REDOX reaction
when both reduction and oxidation occurs
what is oxidation in terms of electrons
loss
what is reduction in terms of electrons
gain
what is reduction in terms of oxygen
loss
what is oxidation in terms of oxygen
gain
how to write ionic equation
show all ions present
how to write half equation
remove spectator ions from ionic equation
chemical name of rust
Hydrated Iron (III) oxide
what conditions do you need for rusting
oxygen and water
5 methods for protecting metal
painting
greasing
coating with plastic
galvanising
sacrificial protection
how does painting work?
stops oxygen and water getting to the iron
how does greasing work?
stops oxygen and water getting to the iron
how does coating with plastic work?
stops oxygen and water getting to the iron
how does galvanising work
coats iron with more reactive metals as a protective layer
how does sacrificial protection work
putting more metal on or connected to iron
eg zinc loses electrons more readily - reacts in pregerence to iron
pros and cons of painting
cheap + simple
scratched/chipped
pros and cons of greasing
movement on chains, cheap
wears off
pros and cons of coating with plastic
durable, cheap
doesn’t work if plastic breaks
pros and cons of galvanising
effective both barrier and SP
expensive
pros and cons of sacrifical proctection
effective, don’t need to cover all iron
expensive, zinc needs replacing
examples of use painting
car bodies, iron railing
examples of use greaing
bike chain
examples of use coating with plastic
dishwasher trays
examples of use galvanising
bin
watering can
examples of use sacrificial protection
zinc blocks on ships