AQA A Level Chemistry- Inorganic chemistry
1/98
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
99 Terms
Atomic radius across Period 3
Decreases
First ionisation energy across Period 3
Increases
Exceptions: Drops at Al and S
Melting points across Period 3
Increases from Na to Si
Decreases from Si to Ar
Small rise at S
Explain why the melting point of aluminium is higher than the melting point of sodium
Bigger charge (3+ compared to 1+)
More free/delocalised electrons
Stronger metallic bonding/stronger (electrostatic) attraction between the ions electrons
Explain why the melting point of sulfur is higher than the melting point of phosphorus
S bigger molecule
So more/stronger van der Waals' forces (to be broken or overcome)
Atomic radius down Group 2
Increases
First ionisation energy down Group 2
Decreases
Melting point down Group 2
Decreases
Exception Ca which increases
Explain the melting point of the elements in terms of the structure and bonding of Group 2 elements
Be and Mg have hexagonal close packed structures
Ca, Sr and Ba have cubic structures
The relative solubilities of the hydroxides, X(OH)2, of the elements Mg-Ba in water
Increase down the group
The relative solubilities of the sulfates, XSO4, of the elements Mg-Ba in water
Decrease down the group
The role of magnesium in the extraction of titanium from TiCl4
Mg is used as a reducing agent
Mg is oxidised to 2+ in MgO when it is heated with TiCl4 to about 1200C in an inert atmosphere
Redox equation of Group 2 elements with water
X(s) + 2H2O(l) -> X2+(aq) +2OH-(aq) + H2(g)
Insoluble Group 2 hydroxide
Mg(OH)2
Insoluble Group 2 sulphate
BaSO4
The use of acidified BaCl2 solution
Test for sulfate ions
If Barium Chloride is added to a solution that contains sulphate ions a white precipitate forms (BaSO4)
The use of Mg(OH)2 in medicine
(in solution as milk of magnesia) to neutralise excess acid in the stomach and to treat constipation
The use of BaSO4 in medicine
In a 'Barium meal' given to patients who need x-rays of their intestines. The Barium absorbs the x-rays and so the gut shows up on the x-ray image. Even though Barium compounds are toxic it is safe to use here because of its low solubility
The use of Ca(OH)2 in agriculture
To neutralise acidic soils (slaked lime)
The use of CaO or CaCO3 to remove SO2 from flue gases
Removed by reacting with an alkali such as CaO or CaCO3 slurry (mixed with
water)
• The process is called wet scrubbing:
CaO(s) + 2H2O(l) + SO2(g) -> CaSO3(aq) + 2H2O(l)
CaCO3(s) + 2H2O(l) + SO2(g) -> CaSO3(aq) + 2H2O(l) + CO2(g)
Explain the trend in electronegativity down Group 7
Decreases because the attracting power of the nucleus is shielded by the inner electron shells increasingly down the group
Explain the trend in the boiling point of the elements in terms of their structure and bonding down Group 7
Increases from F to I, because the larger halogens have greater Van der Waals' forces holding the molecules together, as the relative mass increases down the group
Oxidising ability of the halogens down the group
Decreases going down the group and the more reactive halogen will displace a less reactive halogen from a solution of its ions
Reducing ability of the halide ions
Increases as you go down the Group
How to identify and distinguish between halide ions
Reaction with silver nitrate + dilute nitric acid
F- no precipitate
Cl- white precipitate
Br- very pale cream precipitate
I- very pale yellow precipitate
The nitric acid reacts with, and removes, other ions that might also give a confusing precipitate with silver nitrate
Why ammonia solution is added when testing for halide ions
To confirm the precipitate
Solubility of the silver halides in ammonia
AgCl- precipitate dissolves to give a colourless solution
AgBr- precipitate is almost unchanged using dilute ammonia solution, but dissolves in concentrated ammonia solution to give a colourless solution
AgI- precipitate is insoluble in ammonia solution of any concentration
The reaction of chlorine with water to form chloride ions and chlorate(I) ions
Cl2(aq) + H2O(l) -> ClO-(aq) + 2H+(aq) + Cl-(aq)
The reaction of chlorine with water to form chloride ions and oxygen
2Cl2(aq) + 2H2O(l) -> 4HCl + O2
The use of chlorine in water treatment
Cl is pumped into the water in the final stage of water treatment
This water is stored for about 2 hours to allow disinfection to occur
The benefits to health of water treatment by chlorine outweigh its toxic effects
The reaction of chlorine with cold, dilute, aqueous NaOH and uses of the solution formed
Cl2 + 2NaOH -> NaClO + NaCl + H2O
Reaction of sodium and water
2Na + 2H2O --> 2NaOH + H2 , pH>13
Reaction of magnesium and water
Mg + H2O(g) --> MgO +H2 (FAST)
Mg + 2H20(l) --> Mg(OH)2 + H2 , pH~12
Reaction of Na with oxygen
2Na + 1/2O2 --> Na2O(s)
Vigorous
Reaction of Mg with oxygen
Mg + 1/2O2 --> MgO(s)
Vigorous
Reaction of Al with oxygen
2Al + 1.5O2 --> Al2O3(s)
Slow
Reaction of Si with oxygen
Si + O2 --> SiO2(s)
Slow
Reaction of P with oxygen
P4 + 5O2 --> P4O10(s)
Spontaneously combusts
Reaction of S with oxygen (dioxide)
S + O2 --> SO2(g)
Burns steadily
Melting points of Period 3 oxides
Na2O, MgO and Al2O3 have high melting points due to giant ionic lattice structure creating strong forces of attraction between each ion
SiO has a higher melting point than other non-metal oxides due to giant macromolecular structure
P4O1O and SO3 have low melting points due to simple molecular bonding
Reaction of Na2O with water
Na2O + H2O --> 2NaOH, pH 12-14
Reaction of MgO with water
MgO + H2O --> Mg(OH)2, pH 9-10
Reaction of P4O10 with water
P4O10 + 6H2O --> 4H3PO4 (phosphoric(V)acid)
Reaction of SO2 with water
SO2 + H2O --> H2SO3 (sulphuric(IV)acid)
Reaction SO3 with water
SO3 + H2O --> H2SO4 (sulphuric(VI)acid)
Reaction of Al2O3 with water
Insoluble in water but reacts with BOTH acids and bases to form salts - AMPHOTERIC
Reaction of sodium oxide and hydrochloric acid
Na2O + 2HCl --> 2NaCl + H2O
Reaction of magnesium oxide and sulphuric acid
MgO + H2SO4 --> MgSO4 + H2O
Reaction of magnesium oxide and hydrochloric acid
MgO + HCl --> MgCl2 + H2O
Reaction of silicon oxide and sodium hydroxide
SiO2 + 2NaOH --> Na2SiO3 + H2O
Reaction of phosphorus oxide and sodium hydroxide
P4O10 + 12NaOH --> 4Na3PO4 + 6H2O
Reaction of sulphur dioxide and sodium hydroxide
SO2 + 2NaOH --> Na2SO3 + H2O
Reaction of sulphur trioxide and sodium hydroxide
SO3 + 2NaOH --> Na2SO4 + H2O
Reaction of aluminium oxide and hydrochloric acid
Al2O3 + 6HCl --> 2AlCl3 + 3H2O
Reaction of aluminium oxide and sulphuric acid
Al2O3 + 2H2SO4 --> Al2(SO4)3 + 3H2O
Reaction of aluminium oxide and sodium hydroxide
Al2O3 + 2NaOH + 3H2O --> 2NaAL(OH)4
Cause of transition metal characteristics of elements Ti-Cu
An incomplete d sub-level in atoms or ions
The characteristic properties of transition metals Period 4
• complex formation
• formation of coloured ions
• variable oxidation state
• catalytic activity
Ligand
A molecule or ion that forms a co-ordinate bond with a transition metal by donating a pair of electrons
Complex
A central metal atom or ion surrounded by ligands
Co-ordination number
The number of co-ordinate bonds to the central metal atom or ion
Transition metal
A metal that can form one or more stable ions with an incomplete d sub-level
Monodentate ligands
H2O, NH3 (similar in size and are
uncharged) and Cl− (larger than the uncharged ligands NH3 and H2O)
Exchange of the ligands NH3 and H2O occurs without
Change of co-ordination number
Exchange of the ligand H2O by Cl-
Can involve a change of co-ordination number
Types of ligand
Monodentate- one coordinate bond
Bidentate- two coordinate bonds
Multidentate- more than one coordinate bonds
Haem
An iron(II) complex with a multidentate ligand
How oxygen is transported in the blood
Oxygen forms a co-ordinate bond to Fe(II) in haemoglobin
Carbon monoxide is toxic because
It replaces oxygen co-ordinately bonded to Fe(II) in haemoglobin
The chelate effect
Bidentate and multidentate ligands replace monodentate ligands from complexes
An increase in entropy makes the formation of the chelated complex more favourable
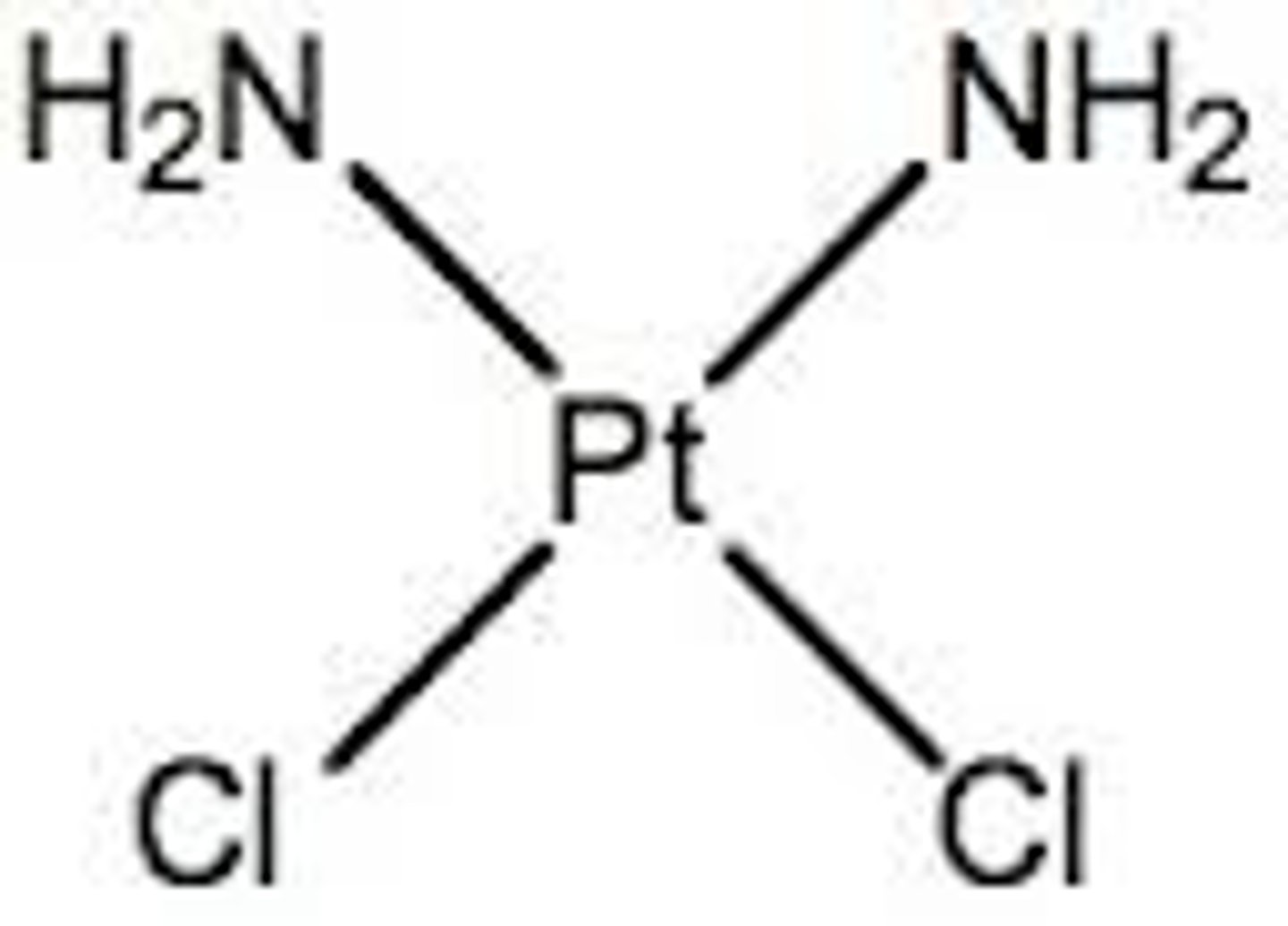
Cisplatin complex
Pt(II) with 2 Cl- and 2 ammonia molecules
Square planar
Cis/Z isomer
Tollen's complex Ag+
Diamminesilver(I) complex
Linear complex [Ag(NH3)2]+
Reduction to metallic silver for aldehyde test
Transition metal ions commonly form octahedral complexes with
Small ligands
Transition metal ions commonly form tetrahedral complexes with
Larger ligands (eg Cl-)
Octahedral complexes can display cis-trans isomerism (a special case of E-Z isomerism) with
Monodentate ligands and optical isomerism with bidentate ligands
Why transition metal complexes are coloured
Colour arises when some of the wavelengths of visible light are absorbed and the remaining wavelengths of light are transmitted or reflected
What happens to transition metal ions when light is absorbed
d electrons move from the ground state to an excited state
The energy difference between the ground state and the excited state of the d electrons in transition metals is given by:
∆E = hν = hc/λ
What causes colour change in transition metal ions
Changes in oxidation state, co-ordination number and ligand alter ∆E which leads to the change
Spectroscopy requires use of
Visible light
Colorimetry
Method used to measure the absorption of light by a sample, the more concentrated it is, the more light will be absorbed
Ligand substitution reaction
One ligand is swapped for another
What happens when ammonium vanadate(V) is reduced by zinc in acidic conditions
Yellow to blue as vanadium(V) is reduced to vanadium (IV)
Blue to green as vanadium(IV) is reduced to vanadium(III)
Green to violet as vanadium(III) is reduced to vanadium(II)
How the redox potential for a transition metal ion changing from a higher to a lower oxidation state is influenced
By pH and by the ligand
The redox equation of Fe2+ with MnO4-
MnO4- + 8H+ 5Fe2+ -> Mn2+ +4H2O + 5Fe3
MnO4- half equation
MnO4- +4e- + 8H+ -> Mn2+ +4H2O
Fe2+ half equation
Fe2+ -> Fe3+ + 5e-
The redox equation of C2O42- with MnO4-
MnO4- + 16H+ C2O42- -> 2Mn2+ +8H2O + 10CO2
Heterogeneous catalyst
A catalyst in a different phase from the reactants and the reaction occurs at active sites on the surface
Support medium
Object used to maximise the surface area of a heterogeneous catalyst and minimise the cost
Examples of heterogeneous catalysts
V2O5 in the Contact process.
Fe in the Haber process
Cost implication of heterogeneous catalysts
Can become poisoned by impurities that block the active sites and consequently have reduced efficiency; this has a cost implication
Homogeneous catalyst
Catalyst in the same phase as the reactants
When catalysts and reactants are in the same phase, the reaction proceeds through an intermediate species
How V2O5 acts as a catalyst in the Contact process
Oxidises SO2 to SO3
SO2 + V2O5 -> V2O4 + SO3
Is oxidised back to original state
V2O5 + 1/2O2 -> V2O5
How Fe2+ ions catalyse the reaction between I− and S2O82-
Fe2+ oxidised to Fe3+
2Fe2+(aq) + S2O82-(aq) -> 2Fe3+(aq) + 2SO42-(aq)
Fe3+ oxides iodine to become Fe2+ again
2Fe3+(aq) + 2I-(aq) -> Fe2+(aq) + I2(aq)
How Mn2+ ions autocatalyse the reaction between C2O42- and MnO4-
MnO₄⁻ + 4Mn²⁺ + 8H⁺ → 5Mn³⁺ + 8H₂O + 10CO₂
2Mn³⁺ + C₂O₄²⁻→ 2Mn²⁺+ 2CO₂
Metal -aqua ions limited to M = Fe and Cu
[M(H2O)6]2+
The acidity of [M(H2O)6]3+ is greater than that of [M(H2O)6]2+
Metal -aqua ions limited to M = Al and Fe
[M(H2O)6]3+
The acidity of [M(H2O)6]3+ is greater than that of [M(H2O)6]2+
Why the acidity of [M(H2O)6]3+ is greater than that of [M(H2O)6]2+
Metal 3+ ions are smaller and have a bigger charge than metal 2+ ions. Metal 3+ ions therefore have a higher charge density than metal 2+ ions. This makes the metal 3+ ions much more polarising than the 2+ ions which means the metal 3+ ions can attract electrons from the oxygen atoms ( of the co-ordinatd water molecules) more strongly thus weakening the O-H bond.It is therefore more likely that the hydrogen atom will be released and the more hydrogen ions the more acidic the solution.