chemical bonds
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Ionic bonding occurs in…
metal and non metal compounds
Covalent bonding occurs in….
Non metal compounds and elements
Metallic bonding occurs in…
metal alloys or compounds
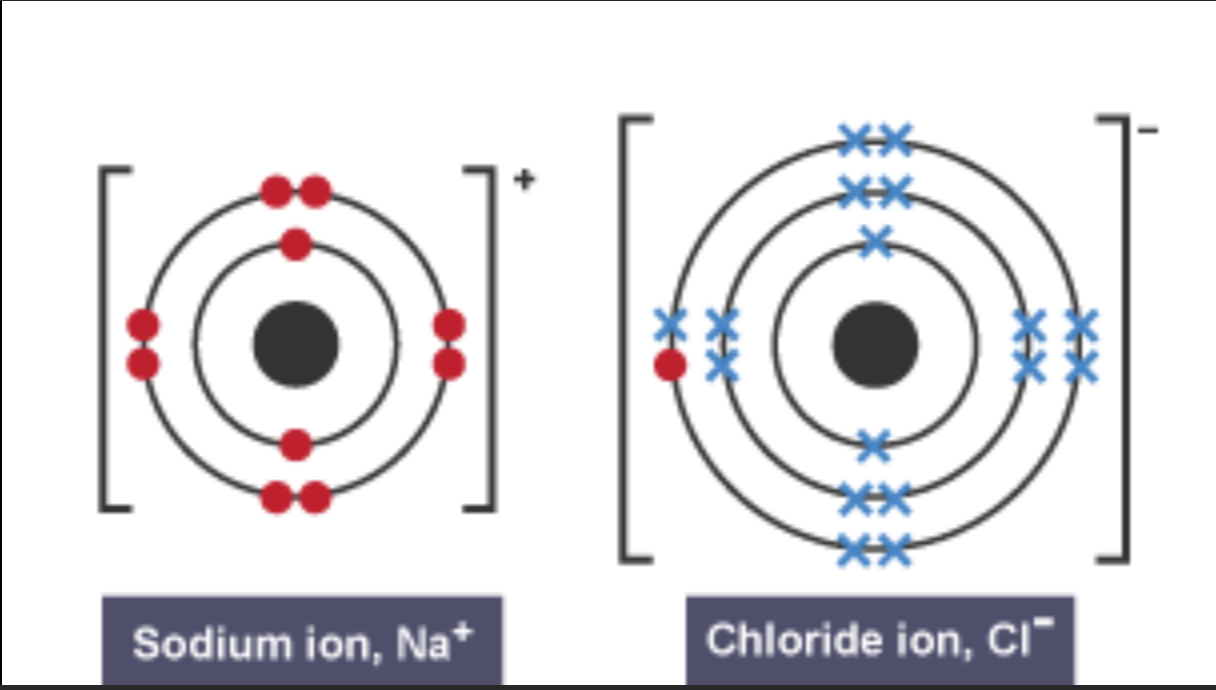
Explain ionic bonding
Between a non metal and metal as they transfer electrons to become more stable
The metal particles lose an electron, becoming positively charged ions
The non metals gain an electron, becoming negatively charged ions
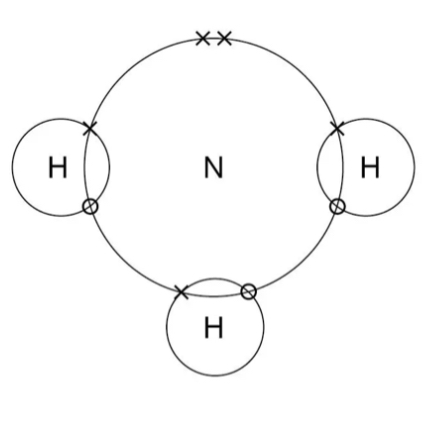
Draw a dot and cross diagram for ionic bonding
Explain covalent bonding
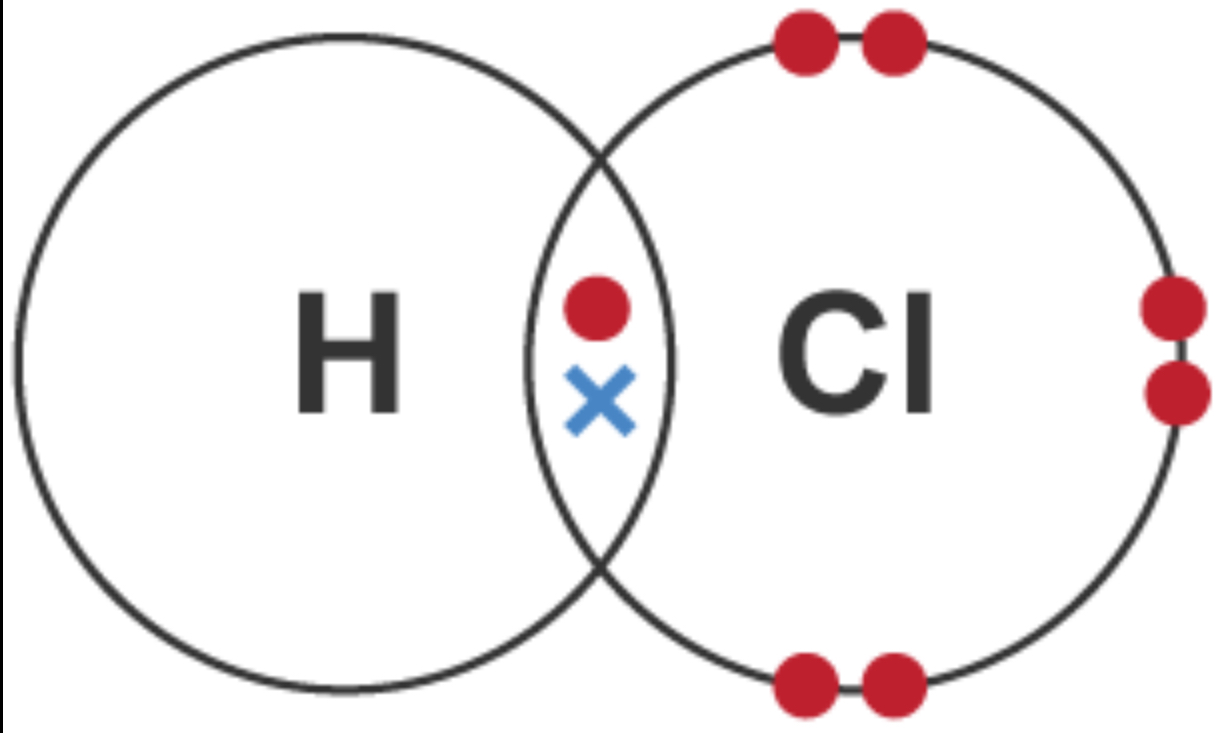
When atoms share one or more pair of electrons
Small molecules (HCl,H²,NH³) have strong covalent bonds within their molecules
Explain metallic bonding
When positive ions (without electrons) ate bonded with delocalised electrons arranged in a regular pattern, free to move throughout the structure
Are Metallica’s bonded structures conductors
Yes because they’re delocalised electrons can carry charge etc
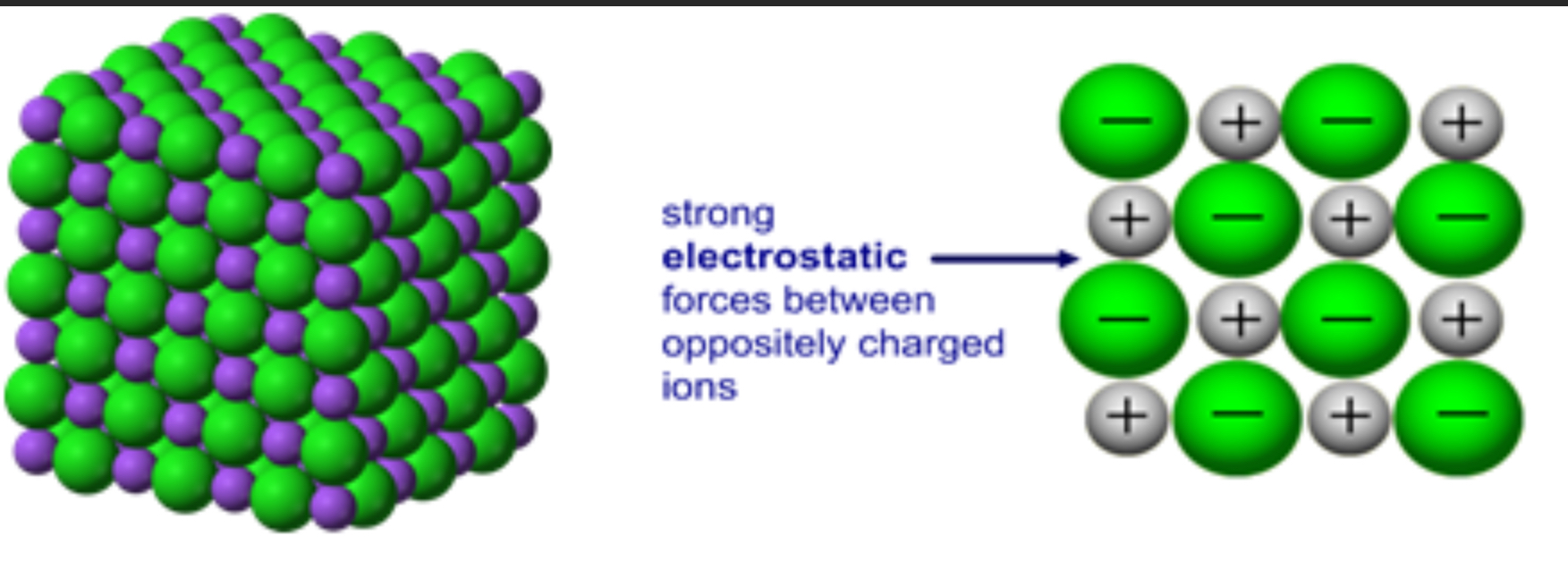
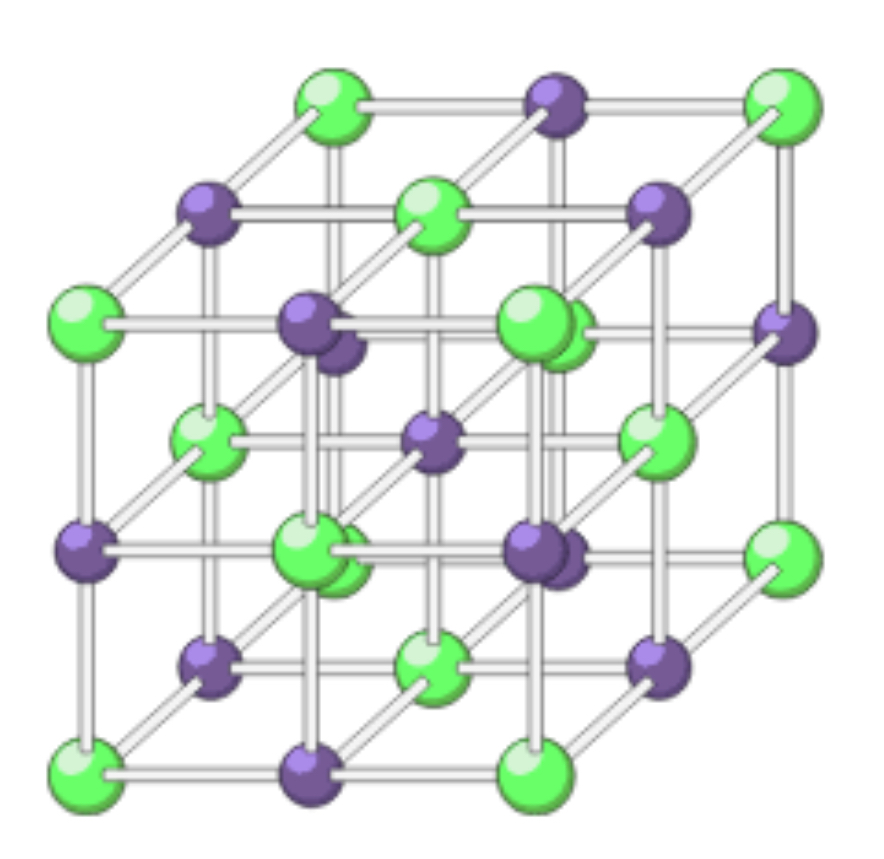
Giant ionic bonds
Held together by strong electrostatic forces between oppositely charged ions acting in every direction as its 3d
Giant ionic bond example
Sodium chloride
Giant ionic structure diagram 1 (lattice)
Giant ionic structure diagram 2 (lattice)
Polymer’s definition
Large covalently bonded molecules
giant covalent structures (macromolecules)
Many atoms covalently bonded in a lattice structure
Giant covalent structures examples
diamond, graphite, silicon, and silicon dioxide
Are metallic bonds strong?
Yes because they have delocalised electrons throughout
Covalently bonded dot and cross diagram
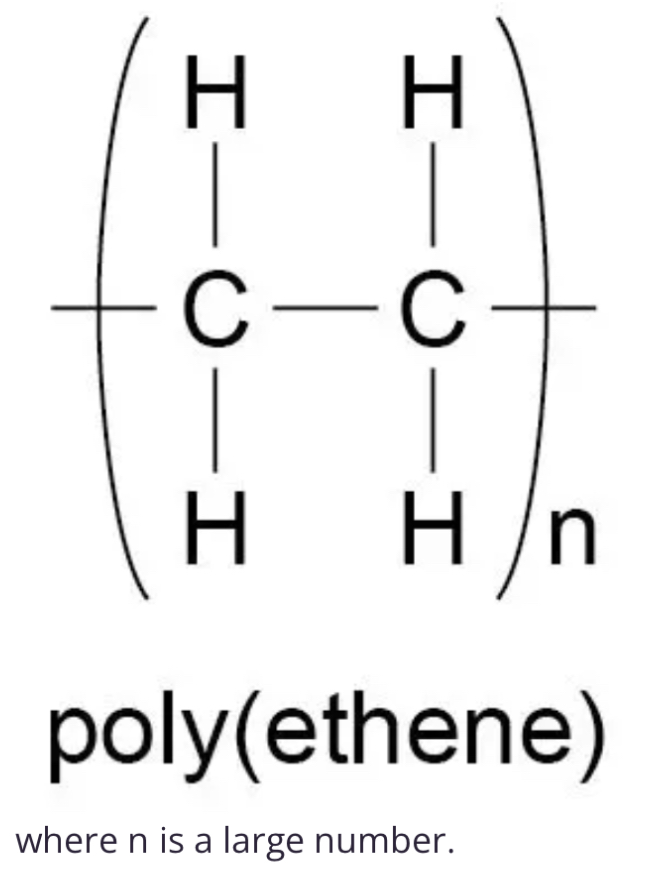
Explain covalent bonds in polymers
Covalent bonds link the repeating units (monomers) in a polymer, forming a long chain molecule. Drawn with a single straight line
Ball and stick diagram
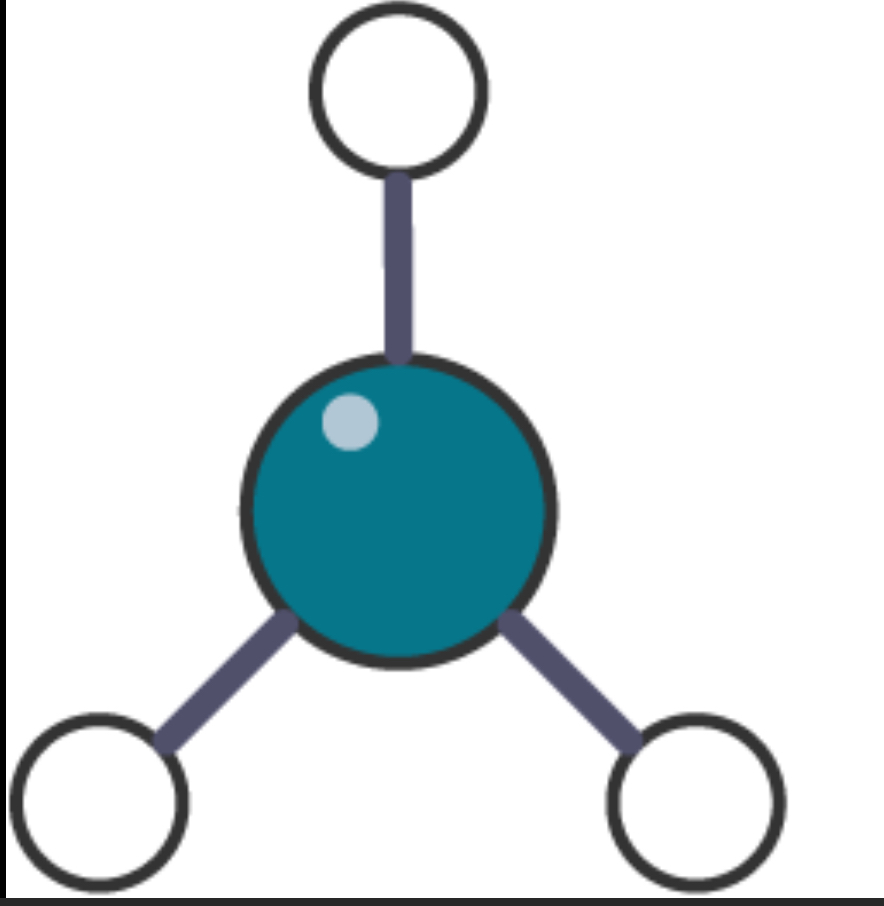
Pros and cons of ball and stick diagrams
Pros
Pros and cons of dot and cross diagrams
Dots and crosses makes it clear where electrons are coming from
Don’t show the shape of the molecule though
Pros and cons of 2d stick diagram (covalent)
Can’t tell which electrons came from where, give no idea of outer electrons not in bonds, don’t give accurate information on shape of molecule
Pros and cons of 3d stick diagrams
Shows the shape
Pros and cons of ball and stick diagrams (ionic 2)
Can clearly see ions in 3 dimensions
Shown as widely spaced when actually they’re packed together, only shows small part of the structure
Space filling diagrams (ionic 1)
Shows how closely packed
Can struggle to see 3d packing, only shows a small part of giant crystal lattice
Empirical formula definition
Simplest ratio of atoms (ions) in a structure
E.g. C²H^6 → CH³
How to find empirical formula
Count the amount of ions of each substance and wrote it down, the simplify
E.g.Na³Cl³ → NaCl
Covalent bond diagram 1
Example polymer structure
Molecular formula
Just count the amount of ions
E.g. H^5Cl^5