PMT 3.4 - Alkenes Flashcards
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
What is an alkene?
What is an alkene?
Unsaturated hydrocarbons with a C=C double bond
What is the general formula of an alkene?
What is the general formula of an alkene?
CnH2n
Why is there no rotation about the C=C double bond?
Why is there no rotation about the C=C double bond?
Due to the π (pi) orbital - electron density above and below the single bond, which holds the carbon atoms in place
Are they more or less reactive than alkanes? Why?
Are they more or less reactive than alkanes? Why?
More reactive, due to high electron density of double bond and the fact the pi-bond is slightly easier to break
What intermolecular forces of attraction do they have?
What intermolecular forces of attraction do they have?
Only van der Waals due to non-polar bonds
Are they soluble in water? Why?
Are they soluble in water? Why?
No, non-polar bonds (van der Waals' < hydrogen bonding)
Name and describe the three kinds of isomers alkenes can have
Name and describe the three kinds of isomers alkenes can have
Chain isomers (branched chains)
Position isomers (C=C on different carbon atom)
Geometric E-Z isomers (Z is when 2 highest atomic number chains are on the same side of the double bond; E is when they're on opposite sides)
Write an equation for the complete combustion of pent-2-ene.
Write an equation for the complete combustion of pent-2-ene.
CH3CH=CHCH2CH3 + 7½O2 → 5CO2 + 5H2O
What is an electrophile?
What is an electrophile?
Electron deficient atoms - lone pair acceptor
What is the most stable type of carbocation intermediate? why?
What is the most stable type of carbocation intermediate? why?
Alkyl groups have a positive inductive effect, so the most stable carbonation is the one bonded to the most other carbon atoms i.e. A tertiary carbocation
Major products will be formed from which kinds of carbocations?
Major products will be formed from which kinds of carbocations?
Tertiary (or the most stable avaliable)
What conditions are needed for the electrophilic addition of H2O to an alkene?
What conditions are needed for the electrophilic addition of H2O to an alkene?
Acid catalyst, usually phosphoric acid
What are the product(s) of the reaction?
What are the product(s) of the reaction?
An alcohol
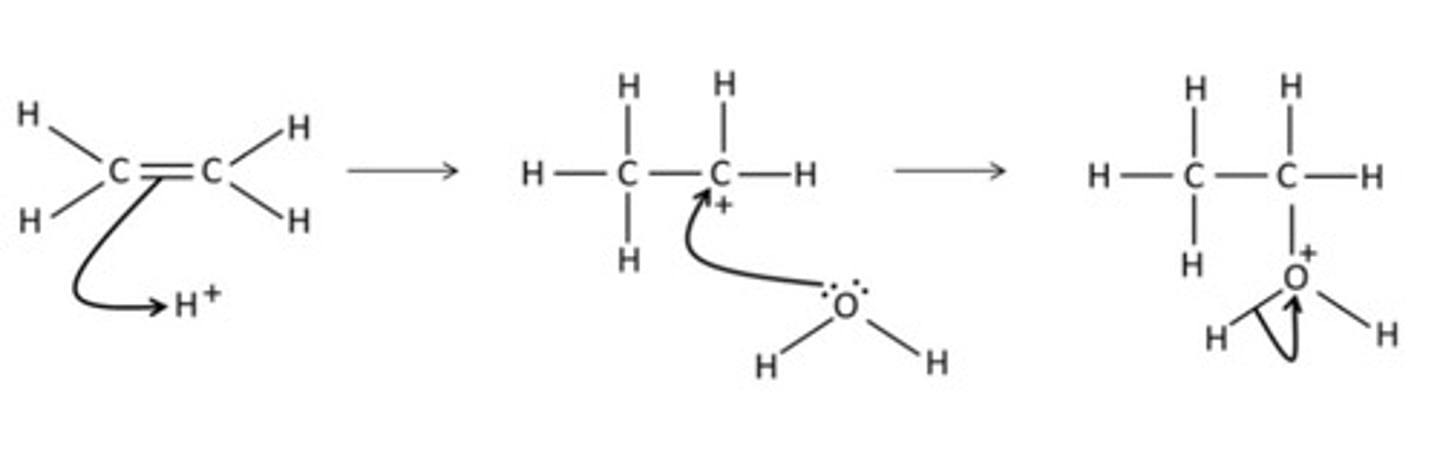
Draw a mechanism for the addition of water to ethene
Draw a mechanism for the addition of water to ethene
What conditions are needed for the electrophilic addition of a hydrogen halide to an alkene?
What conditions are needed for the electrophilic addition of a hydrogen halide to an alkene?
Room temperature
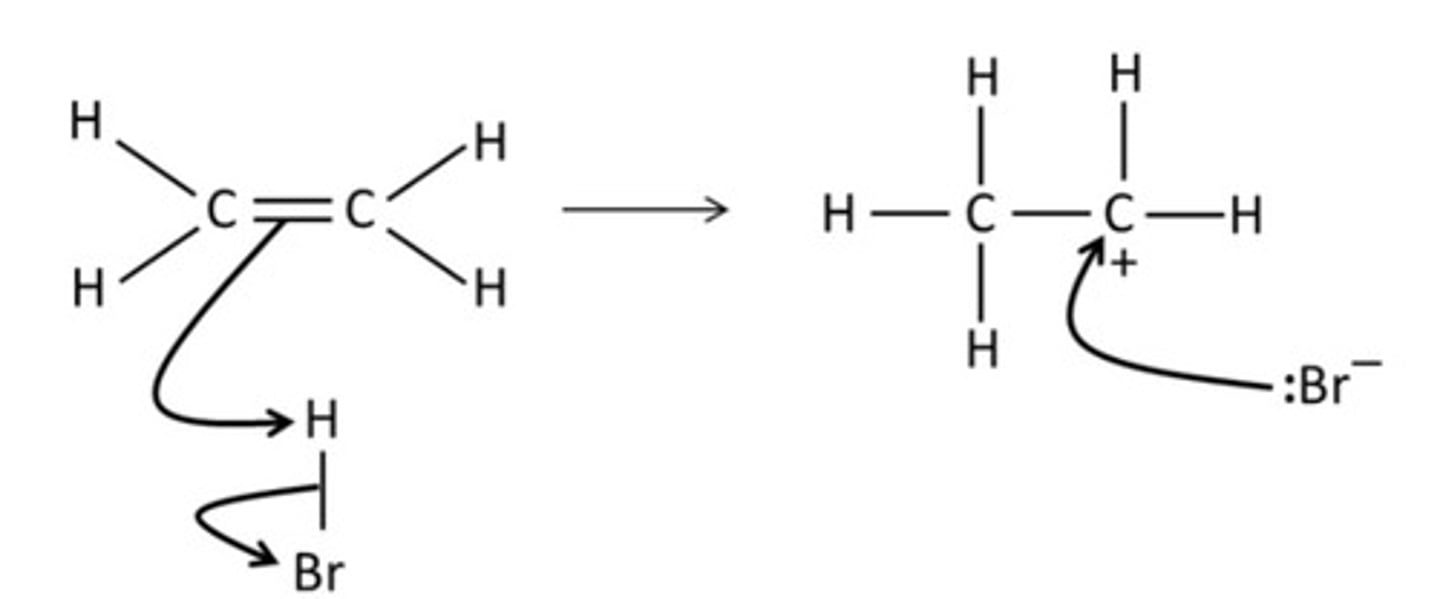
Draw a mechanism for the reaction of HBr and ethene.
Draw a mechanism for the reaction of HBr and ethene.
What conditions are needed for the electrophilic addition of a halogen molecule to an alkene?
What conditions are needed for the electrophilic addition of a halogen molecule to an alkene?
Room temperature and organic solvent
How does a molecule with a non-polar bond react as if it is an electrophile?
How does a molecule with a non-polar bond react as if it is an electrophile?
C=C double bond with a high electron density induces a temporary dipole in the halogen molecule → δ+ atom attracted to double bond
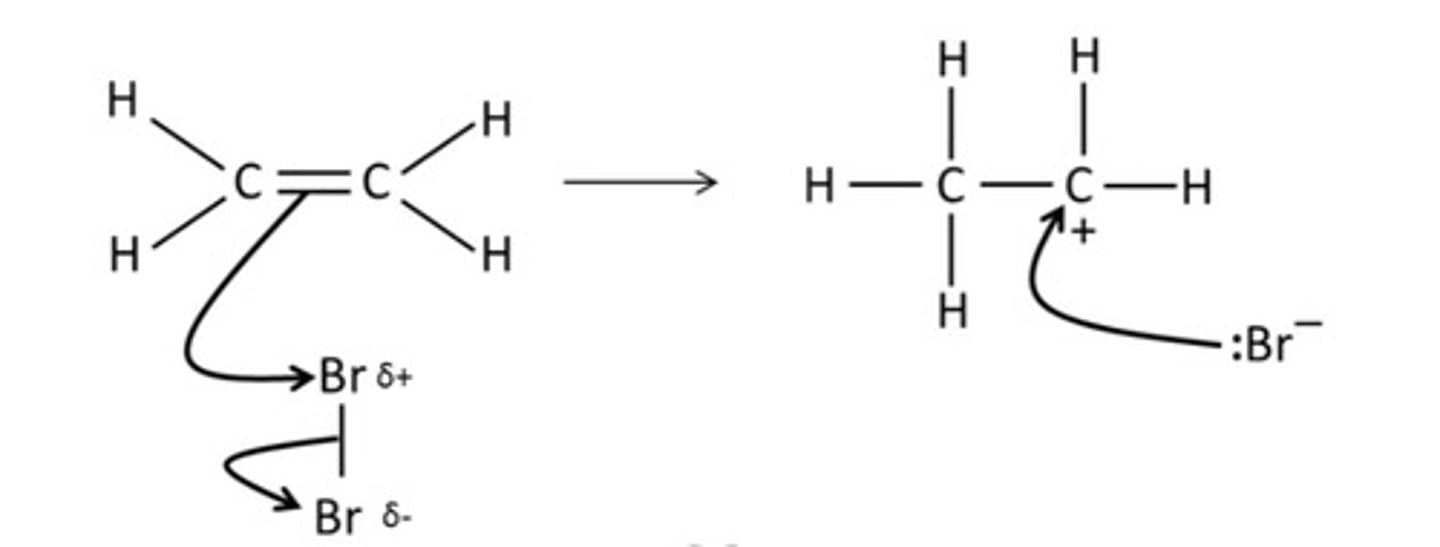
Draw a mechanism for the reaction between bromine and ethene
Draw a mechanism for the reaction between bromine and ethene
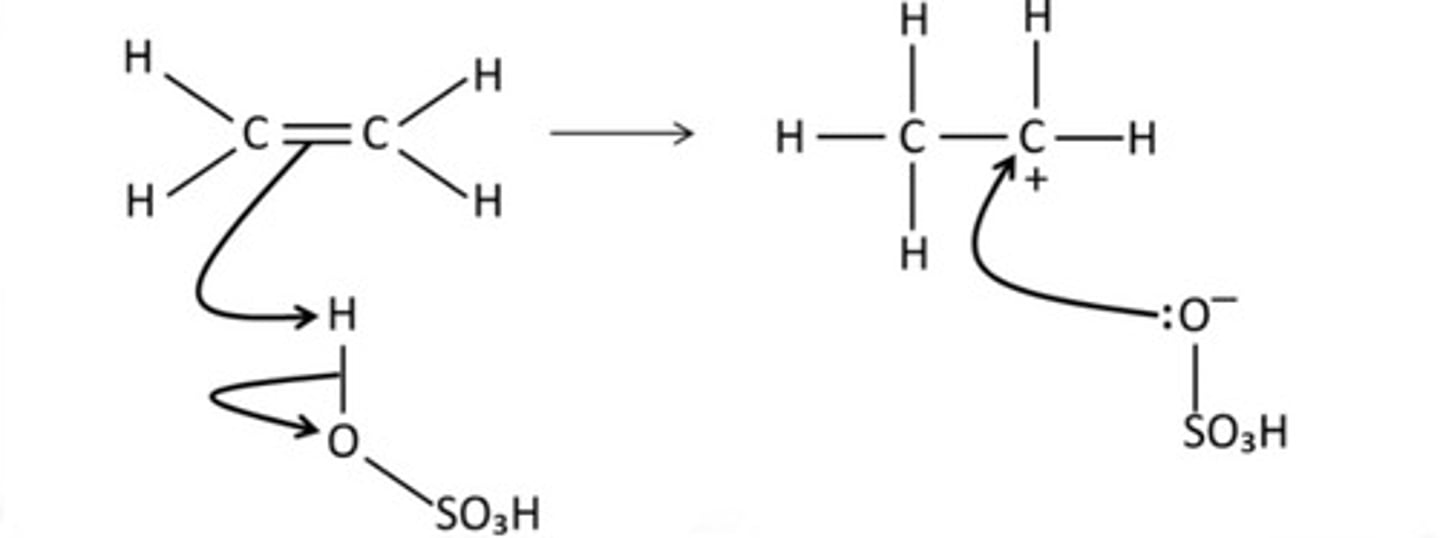
Draw a mechanism for the reaction of sulfuric acid with ethene.
Draw a mechanism for the reaction of sulfuric acid with ethene.
How would you turn the product (from sulfuric acid + ethene) into an alcohol and how does this show that sulfuric acid catalyses the addition of water to an alkene?
How would you turn the product (from sulfuric acid + ethene) into an alcohol and how does this show that sulfuric acid catalyses the addition of water to an alkene?
Add water
H₂SO₄ reforms, showing it catalyses the hydration of alkenes
What is an addition polymer?
What is an addition polymer?
many monomers bonded together via rearrangement of bonds without the loss of any atom or molecule
What are monomers? What form do they usually take?
What are monomers? What form do they usually take?
Molecules which combine to form a polymer
Usually have a C=C bond which breaks to leave a repeating pattern
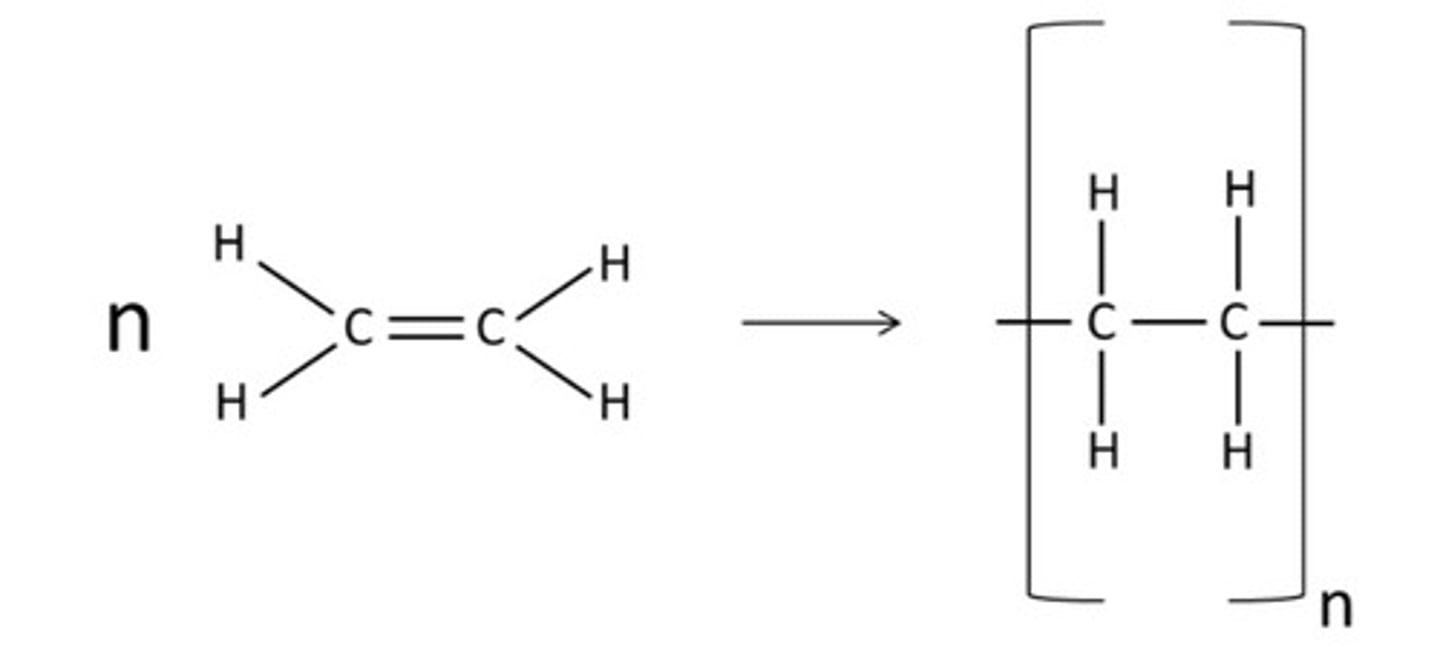
Draw how you would represent the polymerisation of ethene.
Draw how you would represent the polymerisation of ethene.
Give 3 uses of poly(chloroethene) / PVC
Give 3 uses of poly(chloroethene) / PVC
Drainpipes
Vinyl
Aprons
Give two examples of plasticisers
Give two examples of plasticisers
esters and phthalates
What are plasticisers?
What are plasticisers?
Small molecules that get between polymer chains to force them apart and allow them to slide over one another
How do the physical properties of PVC change due to a plasticiser? What applications does this lead to?
How do the physical properties of PVC change due to a plasticiser? What applications does this lead to?
PVC with a plasticiser become flexible, used for aprons
Without a plasticiser, PVC is rigid, used for drainpipes
Why do things containing mainly C-C and C-H bonds not decompose easily?
Why do things containing mainly C-C and C-H bonds not decompose easily?
Bonds are non-polar so are not attacked by enzymes
Why is a lack of biodegradability in compounds with C-C or C-H bonds a problem?
Why is a lack of biodegradability in compounds with C-C or C-H bonds a problem?
Disposal is very problematic
What is mechanical recycling?
What is mechanical recycling?
Where plastics are separated into different types, washed, ground down, melted and re-moulded
What is mechanical recycling used for?
What is mechanical recycling used for?
Soft drinks bottles → fleeces
What is feedstock recycling?
What is feedstock recycling?
Plastics heated to a temperature which break polymer bonds, leaving original monomers that can be made into new plastics
What is feedstock recycling used for?
What is feedstock recycling used for?
Making totally new plastics
What is a problem with recycling?
What is a problem with recycling?
Each time thermosoftening plastics are melted and remoulded, their properties degrade, so they can only be remoulded a limited number of times