orgo reactions
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
53 Terms
what makes a strong nucleophile
neg charge
low electronegativity
what makes a good leaving group
good resonance
electronegativity
larger atom
chactertistcs of something that can bare a negative charge well
what types of carbons does SN2 prefer
primary and methyl (SN2 does toooo much meth)
SN1 prefers secondary and tertiary because that makes for a more stable carbocation
Will strong bases like -OH leave in acidic conditions
No; deprotonation will occur instead
why can’t E2 happen on a methyl group
no free H to make a double bond
what type of reaction is better in a polar aprotic solvent
SN2 and E2 because the nucleophile does not react with the solvent
what type of reaction is better in a polar protic solvent
SN1 and E1 because the rate of the nucleophile attacking does not effect the rate law
Zaitsev’s rule
more substituted double bond is more stable
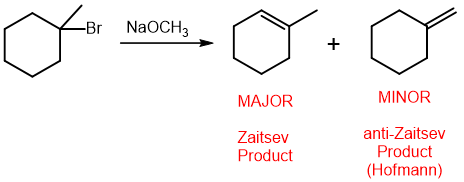
Hofmann’s rule
elimination reactions tend to make the less substituted product
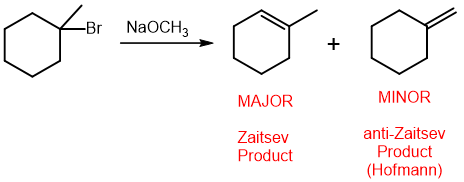
Two ways to make an ether
williamson ether synthesis
condesnsation reaction (if desired product is symmetric)
Williamson ether synthesis
SN2 to make an ether; has selectivity
can’t be done on tertiary carbons because it is SN2
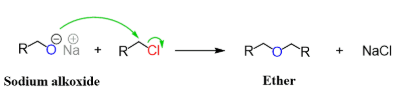
Condensation reaction
two alcohols joined together
only makes symmetrical product
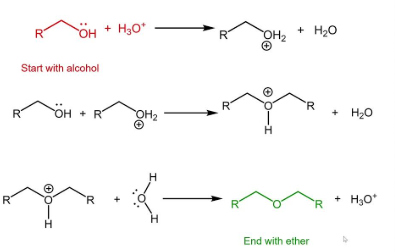
Making a halide from an alcohol
need alcohol and H-X
can do sn1 or sn2 depending on the carbon type
make OH a good leaving group then do sn1 or sn2
if sn1, rearrangements may occur
PBr3 and PCl3
SN2 reaction
inversion of sterochemistry
can’t do on a tertiary carbon
final product just has Br or Cl in place of leaving group with inversion
Halogenation of an alpha carbo in basic conditions
need X2, base, ketone
multiple halogenations occur at all alpha carbons
an alpha carbon is a carbon one off from a double bond
Halogenation of an alpha carbo in acidic conditions
need X2, acid, ketone
one halogenation occurs at a alpha carbons
HOAc is commonly used as the acid
Epoxide ring opening in basic/neutral conditions
nucleophile attacks at less substituted side of ring
Epoxide ring opening in acidic conditions
nucleophile attacks at more substituted side of ring
Formation of epoxides
intramolecular nucleophilic substitution
-O and halide on Carbon chain is a sign
The halide would leave and -O will attack the carbon
Kinetic conditions for deprotonation of alpha carbon
LDA and cold
substrate attaches to less substituted side
kinetic product is formed fast and the reaction is irreversible
Thermodynamic conditions for deprotonation of alpha carbon
strong base and warm
substrate attaches to more substituted side
thermodynamic product is formed slowly and the reaction is reversible
Hofmann Elimination
less substituted product is formed
need Amine group, CH3I, Ag2O, and heat
amine group keeps grabbing methyls until it can’t anymore and then leaves
normal elimination happens
is hydride a base or acid
good base and nucleophile
takes H
Methyl ester synthesis from Diazomethane
need carboxylic acid and CH2N2 (diazomethane)
the H in the carboxylic acid is replaced with a methyl group and N2 is a byproduct
Amine synthesis from Alkyl Halides
ammonia acts as the nucleophile
can’t make primary amines with this because the reaction keeps going
product is a quatenary amine
the alkyl halide is in excess
Electrophilic Addition of Bronsted acid to alkenes
alkene acts as nucleophile and grabs H
carbocation is formed
more stable carbocation is created
acid adds across the double bond
if there’s a chiral center, racemic mixture is made
Acid catalyzed hydration of alkene
double bond is nucleophile in first step and grabs H from acid
water is next nucleophile and attaches to carbocation
water is next nucleophile again and deprotonates water and leaves OH group
water is added across double bond and OH is in more stable position
Electrophilic addition is lowkey another word for…
adding across a pi bond
Electrophilic addition of a bronsted acid to an alkyne
triple bond is two additions
both halides add to same carbon (geminal)
halide adds to more stable carbocation position
Acid Catalyzed Hydration of an Alkyne
triple bond grabs H from acid
water adds to pi bond as nucleophile
water deprotonates other water on big molecule
OH is on the more stable position
tautomerization creates a ketone in the product
1,2 vs 1,4 product
higher temp makes the thermodynamic product (1,4)
makes more substituted alkene (more stable product)
lower temp makes the kinetic product (1,2)
makes more stable carbocation because that reaction is faster
BRRRRR need to get 2 destination fast
this is electrophilic addition of an acid across a double bond
if creating the 1,4 product, look for resonance to create most substituted alkene
Electrophilic addition via a three membered ring
creates enantiomers if product is chiral
concerted mechanism where the electrophile ends up being the point of the epoxide
Electrophilic Addition of crabenes
A carbene is a carbon with two bonds and a lone pair
highly electrophilic
CH2 becomes point of epoxide where double bond is
makes enantiomers if product is chiral
conditions= CH2N2 and hv (light)
Epoxide formation from an alkene via a peroxy acid
MCPBA is the reactant and provides an O
O is the point of the epoxide
product retains stereochemistry
creates enantiomers if product is chiral
Electrophilic Addition with X2 to alkenes
concerted mechanism where X becomes point of epoxide and has a + charge
leaving group is X and attacks the three membered ring
trans stereochemistry across the double bond
Electrophilic addition of X2 to alkynes
no three membered ring intermediate
if there’s an excess of X2 then X adds twice (no more pi bonds)
if there’s 1 equivalence of X2 then X adds once (double bond is created)
cis and trans stereochemistry if 1 equivalence
major product is more stable one
X2 is added across the double bond
Synthesis of Halohydrins
concerted mechanism that begins with X2
X becomes point of the epoxide
reactions happens in water, so water attacks less substituted side of epoxide
water then deprotonates added water
mixed stereochem (Oh and X)
Oxymercuration Reduction of alkene and alkynes
no carbocation
OH goes to more substituted side (water adds across double bond)
anti addition (of OH and H)
reactants = Hg(OAc)2/H2O and NaBH4/NaOH
if it’s an alkyne, keto product is made
if internal alkyne, mix of isomers
like up and down ketone
Hydroboration Oxidation of Alkenes and Alkynes
no carbocation
syn addition (of OH and H)
OH to less substituted carbon (anti markovnikov product)
if alkyne, ketone or aldehyde made depending on where the triple bond is
reactants = BH3/THF and H2O2/NaOH
Catalytic Hydrogenation of Alkenes
addition of H2 across double bond
metal catalyst (Pt/C etc.)
syn addition
double bond basically goes to single bond
Catalytic Hydrogenation of Alkynes with no poisonous catalyst
2 additions of H2 across double bond
triple becomes a single bond
Catalytic Hydrogenation of Alkynes with a poisonous catalyst
poisonous catalyst= Lindlar’s catalyst, pyridine, quinoline
reactions stops after first addition of H2 across double bond
syn addition
Formation of alkynes
NaNH2 and a dihalide
makes triple bond
count carbons!!! to know where triple bond goes
rate law of sn2 and e2
rate= k[substrate][nuc]
rate law of sn1 and e1
rate=k[substrate]
Hydride Reduction of Aldehydes, Ketones, imines, and nitriles
LAH and a separate acid work OR
NaBH4 and a polar protic solvent
Reduces ketone, aldehyde, imines, and nitriles to single bond alcohol or amine group with appropriate amount of H’s
If using LAH, all ketones etc. will be reduced
Addition of Grignard and Alkylithium reagents to aldehydes, ketones, and nitriles
need grignard/Li and H2O as acid work up
If ketone or aldehyde, result is OH group and the added carbon chain
If Nitrile, result is protracted C=N group and the added carbon chain
Wittig Reagents
need aldehyde/ketone and PPh3
Sub oxygen of ketone/aldehyde with the carbon chain part of the wittig
Intermediate has bond connecting O of ketone and P of PPh3
Formation of a Wittig Reagent
need PPh3 and BuLi (a strong base) in a non polar solvent like hexane
Br group leaves off of carbon chain and reaction proceeds through SN2 with PPh3
BuLi deprotonates H and electrons move onto the carbon
Direct 1,2 Addition and Conjugate 1,4 Addition
1,2 (kinetic, irreversible)- quicker reaction and things like grignard or alkylithium or LAH or NaBH4 do it
1,4 (thermodynamic, reversible)- more stable product is made and things not listed up there do it
Cyanohydrin Formation
need HCN and ketone and water
CN attacks a ketone and O of ketone is protonated
Acetal Formation and Hydrolysis
need OR group and ketone or aldehyde
start with protonated form of OR group
OR group is added twice with a series of proton transfers
Never create a negative charge
Imanie and Enamine formation and hydrolysis
imamies forms from a primary amine or ammonia
Enamines form from only secondary amines
Need one of those and a ketone or aldehyde
Ammonia attacks ketone, makes OH2 (good leaving group) then stabilizes N-H group (imine is formed when there is a N=C)
For enamines, secondary amine attacks and product is formed enolate version of enamine is formed