DAT Cellular Respiration (copy)
1/27
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
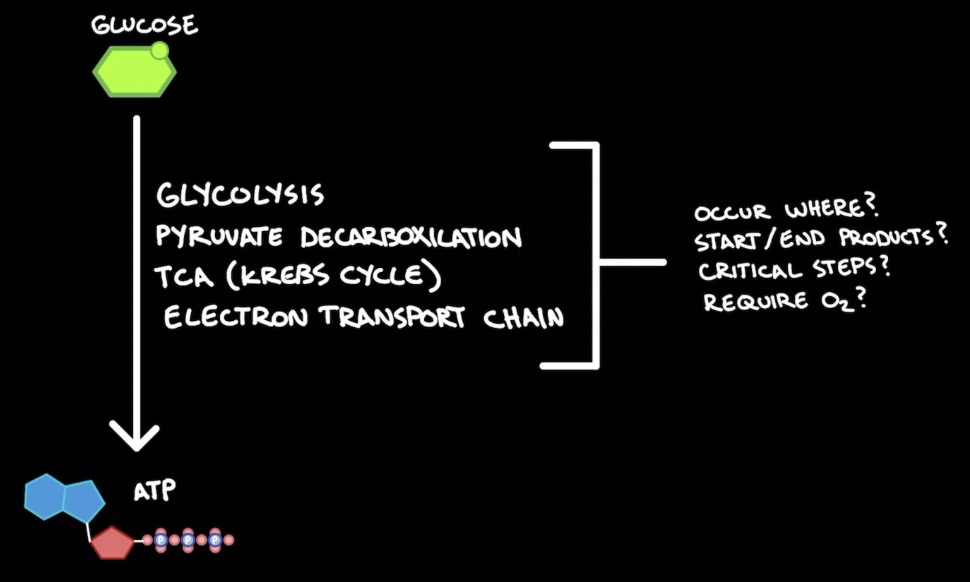
Glucose → ATP
Glycolysis
Pyruvate decarboxylation
TCA (Krebs cycle)
Electron transport chain
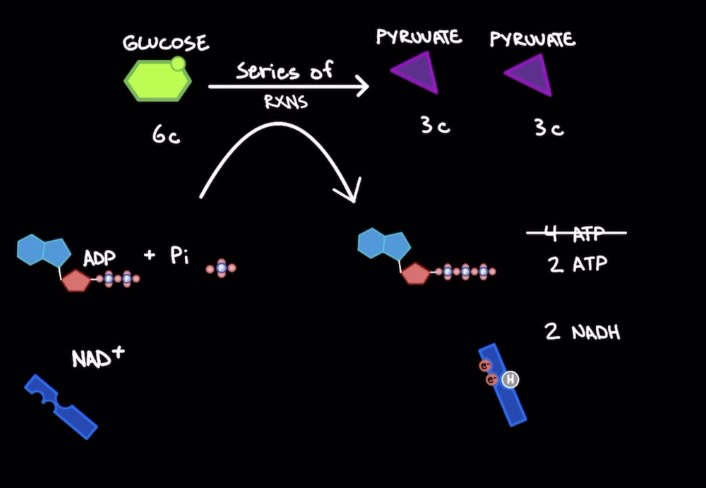
Glycolysis
Break down of glucose
Anaerobic (no O2)
1 glucose mol. (6 Carbons) → 2 pyruvate mols. (3 Carbons)
2 ATP input → 4 ATP output & 2 NADH = 2 net ATP mols. & 2 NADH
Occurs in cytosol (not in organelle)
Steps:
Hexokinase: phosphorylates glucose → Glucose-6-phosphate → irreversible rxn
Phosphofructokinase (PFK): phosphorylates Glucose-6-phosphate → Fructose-1,6-bisphosphate → rate limiting step
Most oxidized form of Carbon
CO2
Waste product of cellular respiration (occurs via oxidation)
Oxidation rxns
ADP + Pi → ATP (oxidized)
NAD+ + FAD+ → FADH2 + NADH (oxidized)
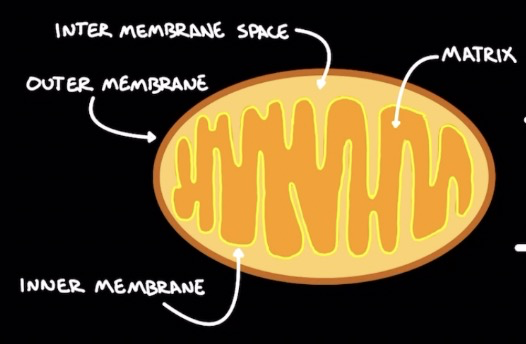
Mitochondria
Double layered
Outer membrane
Intermembrane space: H+ build up
Matrix:
Krebs cycle → produces ATP
β-oxidation to break down fatty acids
Inner membrane: many folds to ↑ surface area → ↑ electron transport chain output
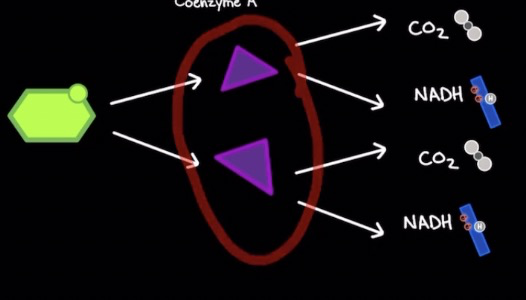
Pyruvate decarboxylation
Occurs in mitochondrial matrix
Aerobic process
2 pyruvate molecules from glycolysis transported into matrix via secondary active transport using protons (doesn’t directly use ATP)
1 Pyruvate + Coenzyme A → Acetyl CoA + 1 NADH + 1 CO2
Pyruvate decarboxylate complex (PDC) catalyzes rxn
How many CO2 and NADH yield from the breakdown of 1 glucose?
2 CO2 + 2 NADH
TCA (Krebs cycle) / Citric Acid Cycle
Occurs in mitochondrial matrix
Aerobic process
1 Acetal CoA + oxaloacetate → citrate
Citrate further oxidizes until oxaloacetate is formed and the cycle repeats
Full cycle yields: 3 NADH + 1 FADH2 + 1 GTP (ATP) + 2 CO2
Electron transport chain (ETC)
Occurs in inner membrane / cristae of mitochondria
Aerobic process
Removes e- from glucose, pyruvate and Acetyl CoA
Oxidative phosphorylation occurs here
Carrier proteins (I, II, III, IV) in inner membrane (electron acceptors): receive electrons from electron transporters (NADH, FADH2) → pump protons against [ ] gradient into intermembrane space to supply energy to ATP synthase
Highly acidic environment in intermembrane space
CoQ (Ubiquinon): can be fully oxidized and reduced during passing of electrons b/w protein complexes
Soluble carrier
Cyt C (Cytochrome C): bound to Iron atom which transfers electrons b/w Complex III and Complex IV for redox rxns
Protein carrier
Used for genetic relations
Final electron acceptor (after electrons have passed though all proteins): Oxygen → combines w/ H+ to form H2O
ATP Synthase: drives protons down the gradient towards matrix (high [ ] → low [ ]) to catalyze ADP + Pi → ATP
pH + Electrical Gradient: Proton Motive Force
If pH of intermembrane space is higher than normal → less H+ → less cellular respiration occurring
NADH creates more ATP (3x) than FADH2 (2x)
NADH pumps more protons to carrier proteins than FADH2 because NADH enters the protein complex earlier than FADH2 and it enters Complex I (FADH2 enters Complex II)
Total glucose produced = 36 ATP in eukaryotes and 38 in prokaryotes (no mitochondria so don’t need to pump NADH into matrix → saving 2 ATP during glycolysis)
![<ul><li><p>Occurs in <mark data-color="yellow"><strong>inner membrane / cristae</strong> of mitochondria</mark></p><p></p></li><li><p>Aerobic process</p><p></p></li><li><p>Removes e- from glucose, pyruvate and Acetyl CoA</p><p></p></li><li><p><mark data-color="red"><strong>Oxidative phosphorylation</strong></mark> occurs here</p></li></ul><p></p><ul><li><p><mark data-color="red"><strong>Carrier proteins (I, II, III, IV)</strong></mark> <strong>in inner membrane (electron acceptors):</strong> receive electrons from electron transporters (NADH, FADH2) → pump protons against [ ] gradient into intermembrane space to <mark data-color="yellow">supply energy to ATP synthase</mark></p><ul><li><p>Highly acidic environment in intermembrane space</p></li><li><p><mark data-color="red">CoQ (Ubiquinon):</mark> can be fully oxidized and reduced during passing of electrons b/w protein complexes</p><ul><li><p><mark data-color="yellow">Soluble carrier</mark></p></li></ul></li><li><p><mark data-color="red">Cyt C (Cytochrome C):</mark> bound to Iron atom which transfers electrons b/w <mark data-color="yellow"><strong>Complex III and Complex IV</strong></mark> for redox rxns</p><ul><li><p><mark data-color="yellow">Protein carrier</mark></p></li><li><p>Used for genetic relations</p><p></p></li></ul></li></ul></li><li><p><mark data-color="yellow">Final electron acceptor</mark> (after electrons have passed though all proteins): <mark data-color="red"><strong>Oxygen</strong></mark> → combines w/ H+ to form H2O</p></li></ul><p></p><ul><li><p><mark data-color="red"><strong>ATP Synthase:</strong></mark> drives protons down the gradient towards matrix (high [ ] → low [ ]) to catalyze ADP + Pi → ATP</p><ul><li><p><mark data-color="yellow">pH + Electrical Gradient: <strong>Proton Motive Force</strong></mark></p></li><li><p>If pH of intermembrane space is higher than normal → less H+ → less cellular respiration occurring</p></li></ul></li></ul><p></p><ul><li><p>NADH creates more ATP (3x) than FADH2 (2x)</p><ul><li><p>NADH pumps more protons to carrier proteins than FADH2 because NADH enters the protein complex earlier than FADH2 and it enters Complex I (FADH2 enters Complex II)</p></li></ul></li></ul><p></p><ul><li><p><mark data-color="yellow">Total glucose produced = 36 ATP in eukaryotes and 38 in prokaryotes (no mitochondria so don’t need to pump NADH into matrix → saving 2 ATP during glycolysis)</mark></p></li></ul>](https://knowt-user-attachments.s3.amazonaws.com/cc5a296811364aa5a49731ec032ed53f.jpeg)
Oxidative phosphorylation
Process of ADP → ATP from NADH and FADH2 via passing of e- through various carrier proteins in the electron transport chain
Electron carriers
NADH and FADH2
What are the products of 1 Glucose molecule that has only undergone the Krebs cycle?
1 glucose → 2 pyruvate → 2 acetyl CoA → 6 NADH + 2 FADH2 + 2 GTP + 4 CO2
Fermentation
Occurs when [O2] is too low to carry out aerobic processes in mitochondria
NAD+ formation prioritized to form NADH
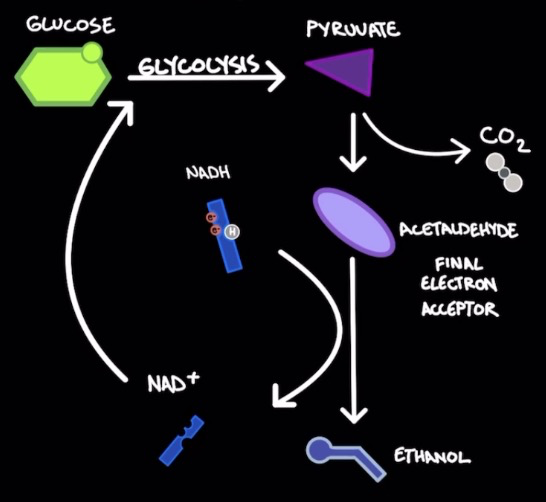
Alcohol fermentation
Lactic Acid fermentation
Alcohol fermentation
Fungi (yeast), bacteria, plants
Reduces pyruvate (from glycolysis) → acetaldehyde + CO2 → ethanol (by product) in a process that oxidizes NADH → NAD+
Acetaldehyde is the final e- acceptor from NADH
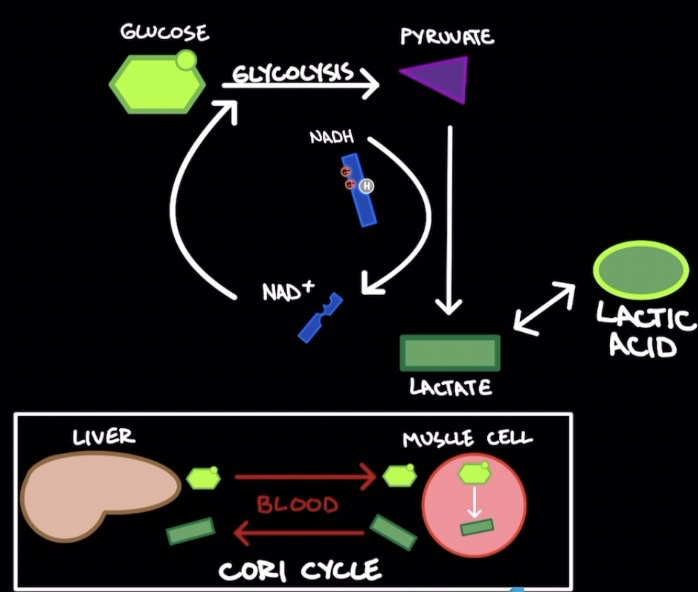
Lactic Acid Fermentation
Occurs in muscle cells
Use glycolysis to produce 2 pyruvate mols.
Pyruvate reduced to lactate (by-product) → oxidizes NADH → NAD+
Lactate (weak base) → Lactic acid (strong acid)
Cori Cycle: lactate from muscle cells transported into blood stream → liver → converted to glucose → blood stream → used to generate ATP through glycolysis
Catabolic rxns
Releases energy by breaking down large molecules into smaller molecules
Anabolic rxns
Requires energy to build molecules from smaller molecules
Cellular metabolism
Anabolic & Catabolic rxns
What happens if a cell does not have glucose?
Uses other carbohydrates
Lipids
Proteins (last resort)
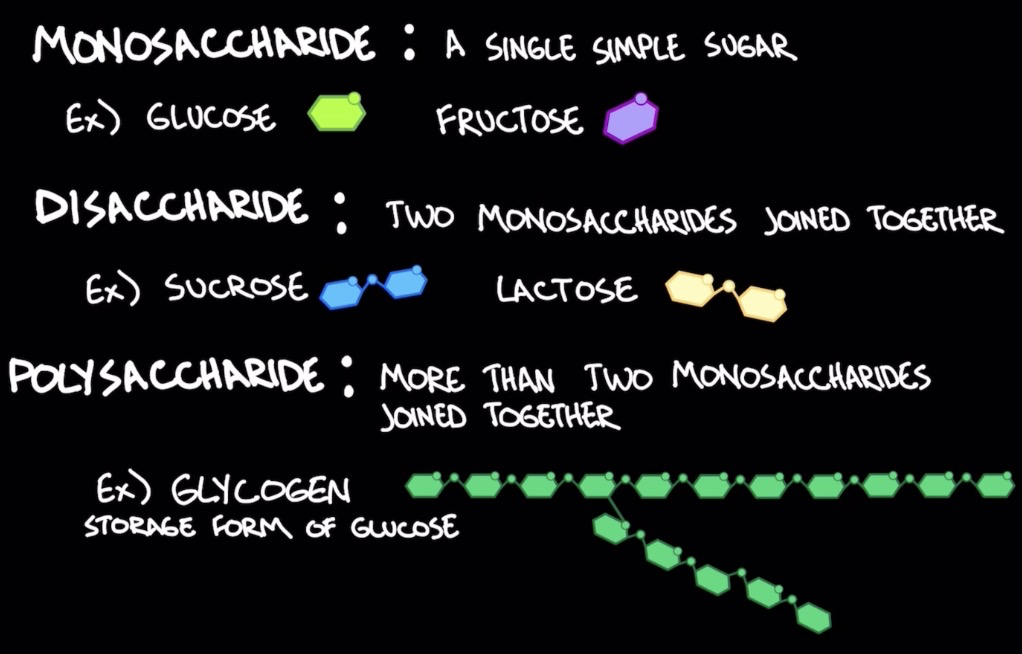
Carbohydrates as a source of energy
Glycogen (polysacc.): storage for glucose
found mainly in muscle and liver cells
Glycogenesis: formation of glycogen (glucose → glycogen)
Glycogenolysis: break down of glycogen (glycogen→glucose)
Glucose-6-phosphate main molecule for rxn
Regulated by Insulin and glucagon
First broken down in mouth → stomach → duodenum → small intestine
Disaccharides hydrolyzed into monosaccharides → converted to glucose or glycolytic intermediates
All cells can store glycogen but only skeletal muscle and liver cells can store large amounts
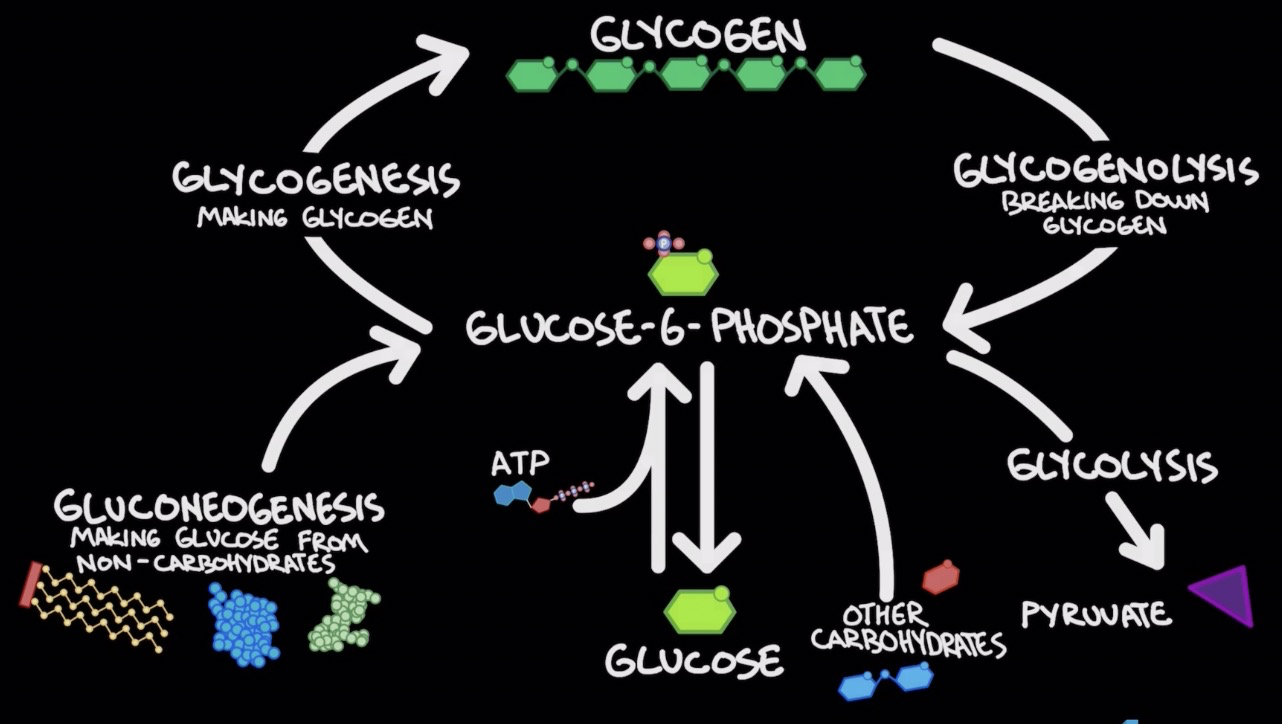
Gluconeogenesis
Forming glucose from non-carbs
Occurs in liver and kidney
Why is phosphate added to a glucose molecule?
To keep the molecule w/n the cell and prevent it from diffusing out of the cell
Insulin
Endocrine hormone
Released by pancreas when glucose ↑
Triggers cells to:
make glycogen from glucose for storage
undergo glycolysis to form ATP → activates Phosphofructokinase (R.D.S)
Glucagon
Released by pancreas when glucose ↓
Similar to epinephrine (triggers formation of glucose)
Triggers cells to:
Glycogenolysis to form glucose
Inhibit glycogenesis to inhibit glycogen production
Lipids as a source of energy
Long hydrocarbon chains that are highly reduced → have more energy than carbs
Triglycerides: 1 glycerol backbone bound to 3 fatty acid chains
Lipases in adipose tissue are hormone sensitive (e.g., to glucagon)
Lipolysis: break down of lipids into glycerol and the fatty acids by lipase enzymes
Glycerol → Glyceraldehyde 3 Phosphate (DAP/G3P/PGAL) → enters glycolysis
Fatty acid chains activated by 2 ATP → β-oxidation of saturated FA in mitochondrial matrix (breaks 2 carbons at the β position) → 1 NADH + 1 FADH2 and 1 Acetyl CoA → citrate in Krebs cycle → 120 ATP generated
β-oxidation of unsaturated FA → 1 less FADH2 for each double bond
Lipids combine w/ soluble proteins → lipoproteins (contain Apoproteins)
Classified by density (fat : protein ratio)
B/w meals, most lipids in plasma are in the form of lipoproteins
Lipoproteins large and less dense when ratio is also large
Chylomicrons: first fat transporters to leave enterocyte and enter lacteals (small lymphatic vessels)
LDL (low protein density): unhealthy due to high fat content
HDL (high protein density): healthy cuz transport fat away from tissues → liver → cholesterol for bile → expelled during digestion
What carries fatty acids in blood?
Albumin
Lipid digestion
Stored as adipose tissue
Only broken down in duodenum:
Bile released from gall bladder to emulsify fats & pancreatic lipase to break down lipids into FA chains and monoacylglycerides
Absorbed into enterocytes of small intestine
Reassembled into triglycerides, and then, along with cholesterol, proteins or phospholipids → packaged into chylomicrons
Chylomicrons move to lymph capillary → circulatory system
Proteins as a source of energy
Excess amino acids used for energy
Oxidative deamination: removal of amino group to form metabolic intermediates (Acetyl CoA, pyruvate, Oxaloacetate)
Directly removes ammonia from AA
Deamination in liver
By-product: NH3 (ammonia) → urea → excreted as urine in mammals
Uric acid in insects, birds, reptiles
Digestion occurs in stomach: pepsin breaks down proteins into polypeptides
In the small intestine: trypsin breaks down specific polypeptides into amino acids