2.2 Atomic Masses
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
Calculate the atomic mass of rubidium if 72.17% of naturally occurring rubidium atoms have a mass of 84.9118 and 27.83% have a mass of 86.9092
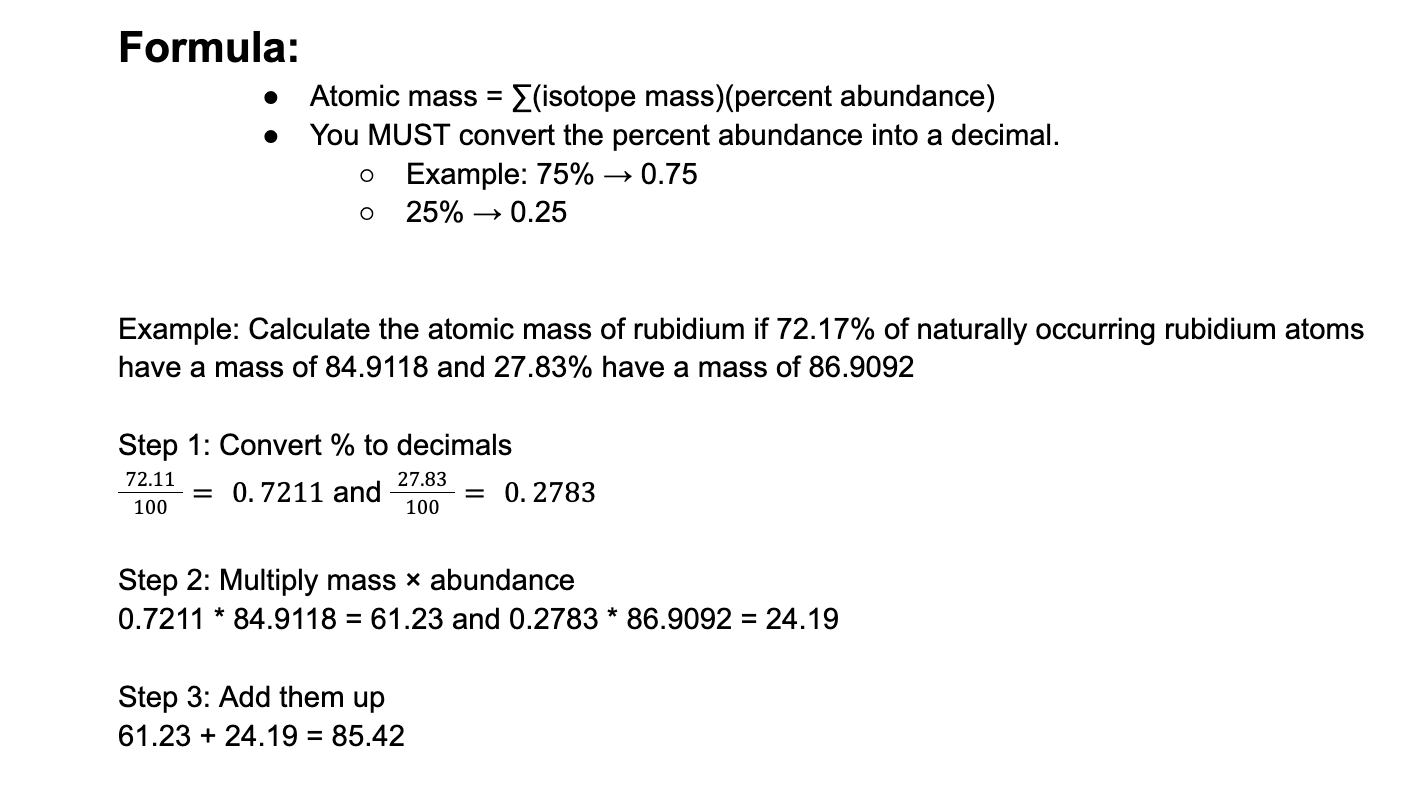
The atomic masses of 20Ne (90.48 percent), 21Ne (0.27 percent), and 22Ne (9.25 percent) are 19.9924356, 20.9938428, and 21.9913831 amu, respectively. Calculate the average atomic mass of neon. The percentages in parentheses denote the relative abundances
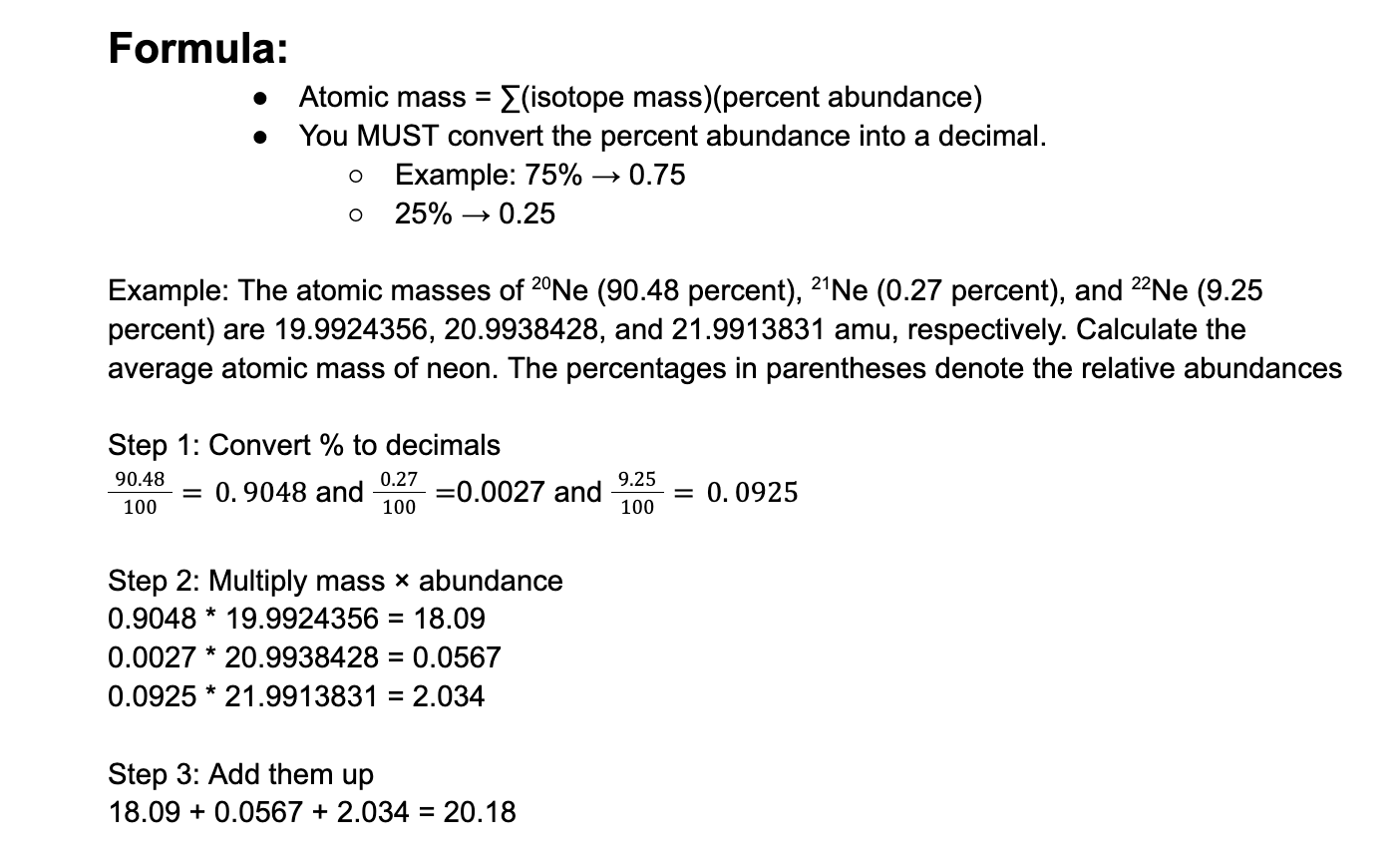
In a combustion reaction, 46.0 g of ethanol reacts with 96.0 g of oxygen to produce water and carbon
dioxide. If 54.0 g of water is produced, what mass of carbon dioxide is produced?
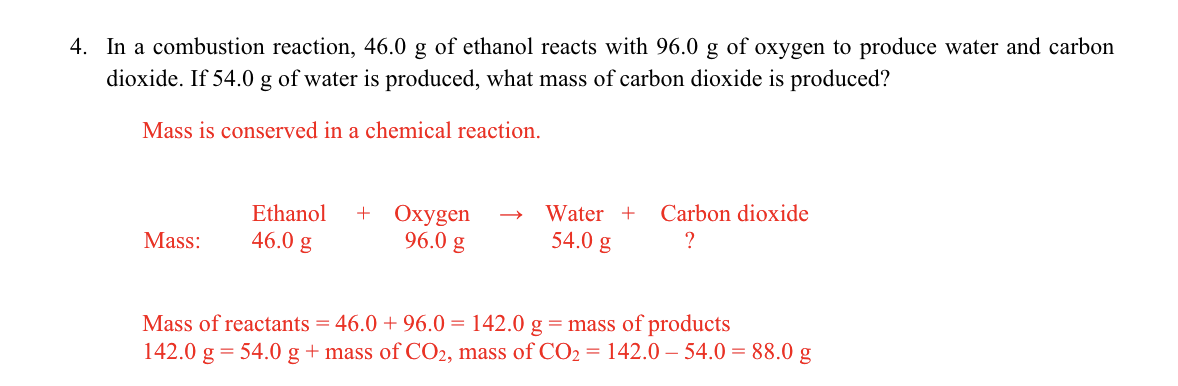
Silicon has an atomic mass of 28.08 amu. Silicon has three naturally occurring isotopes, Si-28 is 92.23% abundant (mass= 27.97), Si-29 is 4.68% abundant, (mass= 28.97). What is the mass of the third naturally occurring isotope of Silicon, and what is its relative abundance?"

In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor.
Hydrogen + Oxygen —> water
If you burn 56.6g of hydrogen and produce 506 of water, how much oxygen reacted?
506 - 56.6 = 449
Element X has an average atomic mass of 10.50 amu.
It has two known isotopes:
X-10 has a mass of 10.00 amu and is 60% abundant.
X-12 has a mass of 12.00 amu and is 25% abundant.
There is a third isotope, X-11.
What is its mass and abundance?
