Nuclear power
1/76
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
77 Terms
Nuclear history
•On March 11, 2011, a huge earthquake struck Japan
•The earthquake’s damage was not devastating
•But, a 50-foot-high tsunami swept over seawalls
•20,000 died, 1 million buildings were damaged, and economic losses exceeded $300 billion
•11 of 55 nuclear power plants shut down automatically
•The Fukushima Daiichi power station, with six nuclear reactors, was hit
•Seawater cut off electric power and destroyed the backup generators
Fukushima Daiichi Power Plant (1 of 2)

Fukushima Daiichi meltdown
•With no cooling water, three of the six reactors suffered meltdown and explosions from overheated fuel rods
•Releasing radioactive fission products into the air
•75,000 people in a 12-mile radius were evacuated
•Seawater was used to cool the reactor vessels and pools holding spent fuel rods
•The exclusion zone has become a “dead zone”
•A fabric cover prevented more airborne radiation
•It took six months to bring temperatures below boiling
•It took until the end of 2011 to control the heat
Impact of nuclear meltdowns
•Although nobody has died from radiation, governments from all over the world are examining nuclear power
•Japan: canceled plans to build 14 new plants
•China: moving full speed ahead on new plants
•But will assess safety conditions at existing plants
•Germany and Switzerland: will phase out nuclear
•Italy: plans to build new facilities are on hold
•United States: will review the safety of all its plants
•Nuclear power’s risks are small
•But accidents have severe consequences
Nuclear power in place of fossil fuels
Long Island shoreham nuclear plant
•Was completed and licensed at a cost of $5.5 billion in 1984, commissioned 1985-1989
•After operating only 32 hours, it was dismantled
•People could not be evacuated if an accident happened
Current nuclear situation in the US
•At the end of 2014, 100 nuclear reactors were operating in the United States
•The Watts Bar Unit 1 reactor (in Tennessee) was the last one completed, in 1996
•The Watts Bar Unit 2 reactor will be the first reactor completed in the 21st century
•U.S. plants generate 20% of U.S. electrical power
•Given the government incentives for nuclear energy, nuclear generating capacity will increase
Currently, new reactors are replacing aging ones
Fossil fuel problems
•Coal: generates the most greenhouse gases and pollution
•Oil and natural gas: are limited
•Oil: vital for transportation
•Hydroelectric: already heavily developed
•Wind and solar: provide only a small amount of electricity
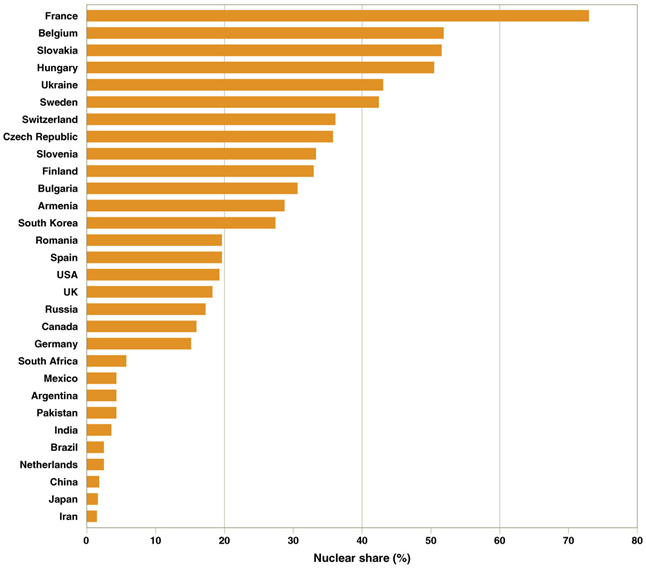
Global nuclear power use
Nuclear share of electrical power generation
Less fossil fuel reserves = more nuclear power
France is greatest
Belgium 2nd
Slovakia 3rd
China/Japan/Iran least
Objective of nuclear power
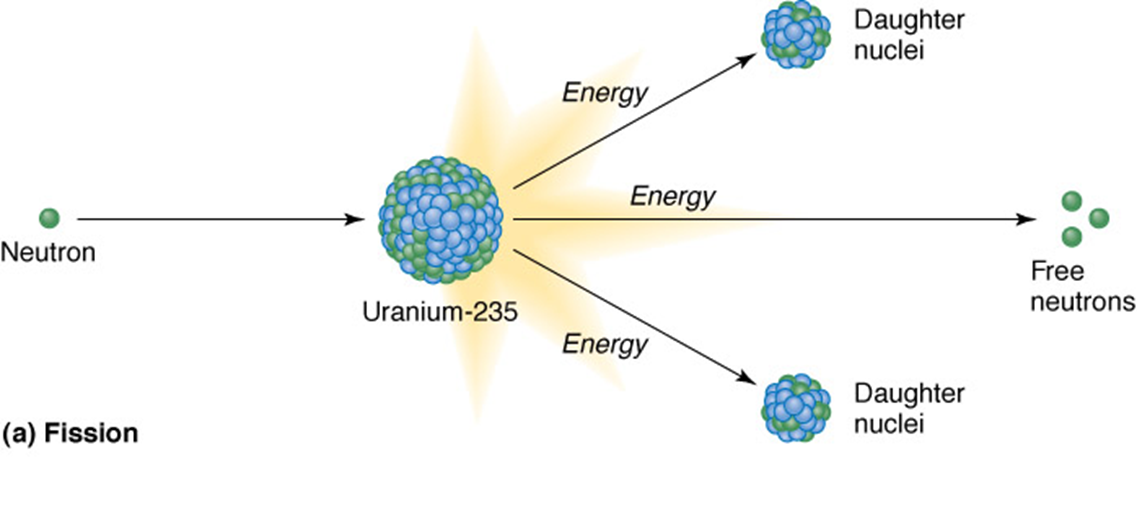
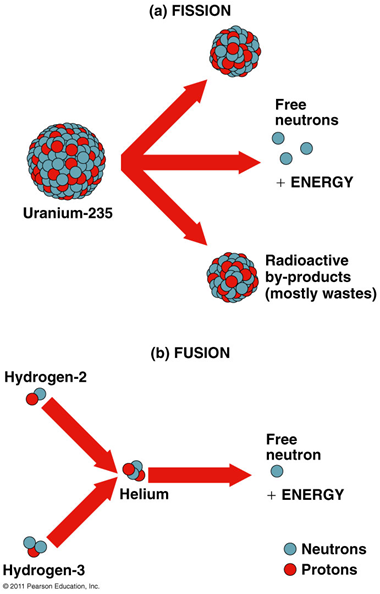
Fission
a large atom of one element is split into two atoms of different elements
•The products of both have less mass than the starting material
•The small mass is multiplied by the speed of light squared, resulting in a tremendous release of energy, E = mc2
Fusion
two small atoms join to form a larger atom of a different element
•The products of both have less mass than the starting material
•The small mass is multiplied by the speed of light squared, resulting in a tremendous release of energy, E = mc2
Ionizing r
Hydrogen isotype
most abundant element in the Universe
11H stable isotope; atomic numbern+pH
21H (also 21D) Deuterium, stable isotope, 0.03%
31H (also 31T) Tritium, radioactive, t1/2 = 12.3 years
31H -----à 32He + b- + n (neutrino) + 18.6 KeV
Maximum distance b (electron) particle travels in air: 6mm
Beta decay
Not very powerful energy
: 10n ----à 11p+ + b- + energy
11p+ , Proton [2 Up Quarks (2/3+) + 1 Down Quark (1/3-)]
10n, Neutron [1 Up Quark + 2 Down Quarks]
Carbon isotope
126C stable isotope, most abundant, 99.9%
136C stable isotope
146C radioactive, t1/2 = 5,730 years
146C -----à 147N+ + b- + n (neutrino) + 156 KeV
Maximum distance b particle travels:
Air: 22cm
Water (or tissue): ~2.5mm
Uranium fission
•Uranium atoms that undergo fission release additional neutrons, causing additional fission and heat.
Example of one of many decay pathways of 23592U
23592U -----à 22286Rn + 42He + 4.678 MeV + g 186 KeV
t1/2 = >700 Million years
Example of what takes place in a Nuclear Reactor with high energy Neutrons (10n):
23592U + 10n -----à 23692U -----à 14156Ba + 9236Kr + 3 10n + 202.5 MeV
t1/2 = msec
23692U -----à 23290Th + 42He
t1/2 = 23.4 Million years
Uranium fuel source
•occurs naturally in the Earth’s crust
•It exists isotopes, but uranium-238 (238U) and uranium-235 (235U) are useable in reactors
•Different mass numbers come from different numbers of neutrons (238U = 146, 235U = 143 neutrons)
•235U readily undergoes fission, but not 238U
Isotope
contain different numbers of neutrons but the same number of protons and electrons
Nuclear fission
occurs when a neutron hits the nucleus of 235U at just the right speed
•Some atoms of 235U undergo radioactive decay and release neutrons
•These neutrons can hit other 235U atoms, producing highly unstable 236U
•236U undergoes fission into lighter atoms (fission products)
•More neutrons are given off, releasing lots of energy
•This domino effect causes a chain reaction
Nuclear bomb
•When 235U is highly enriched, spontaneous fission of an atom triggers a chain reaction
•Nuclear weapons have small amounts of pure 235U
•Or other fissionable material
•Two or three neutrons from a spontaneous fission cause two or three other neutrons to undergo fission, etc. producing a expanding chain reaction
•The whole mass undergoes fission in a fraction of a second
•Releases all energy in one huge explosion
Milling
Ore crushed, treated chemically, turned into yellowcake
Yellowcake
80% UO_2 (oxidized uranium)
purified and enriched
Enrichment
separates 235U from 238U to produce 3%–5% 235U (the rest is 238U)
•Technical difficulties prevent less developed countries from advancing their nuclear programs
Nuclear reactor
•A nuclear reactor has a continuous chain reaction
•But does not amplify it into an explosion
•Control is through enriching uranium to 3–5% 235U, slowing down emitted neutrons (with water) and remaining below a critical mass (density)
•Faster neutrons absorbed by 238U convert it to 239Pu
•Plutonium also undergoes fission and releases energy and can be used in Nuclear Reactors
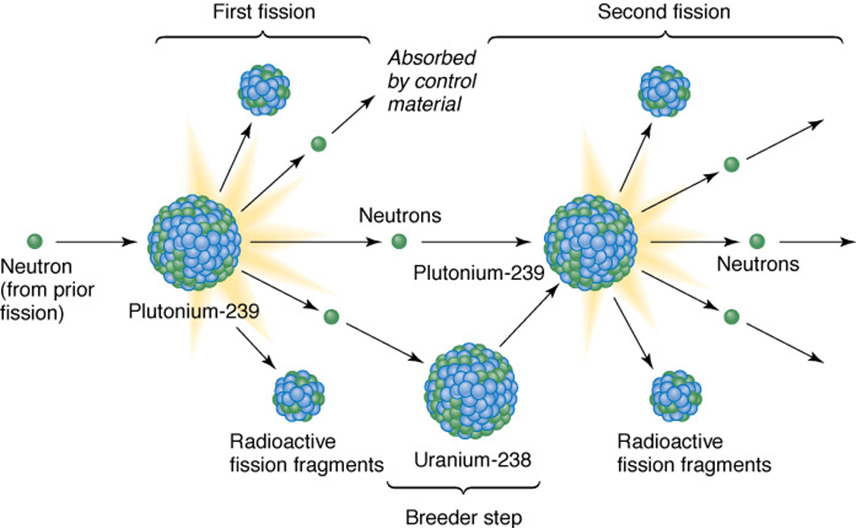
Breeder reactor
use 238U to make 239Pu
•Breeder reactors use chain reactions to utilize uranium235
•Fast-neutron reactors: use the extra two neutrons created during fission of 235U to convert nonfissionable 238U to 239Pu
•Increases nuclear fuel reserves more than 100 times
Light-water reactors (LWR)
uranium moderator is near-pure water
U235
crucial for nuclear power and weapons because its atoms can be easily split, a process called fission. It occurs naturally but makes up only about 0.7% of natural uranium, so it must be enriched to a higher concentration for use in most nuclear reactors.
U238
Most common isotope of uranium. not stable enough for fission
Nuclear reactor control
•Enriched uranium is arranged in a suitable geometric pattern surrounded by the moderator (water)
•Uranium pellets are inserted into long metal tubes (fuel elements, fuel rods)
•Fuel rods are placed close together to form a reactor core
•The core is inside a water-holding vessel (the moderator and coolant)
•Neutron-absorbing fission products accumulate in the rods
•They slow down the rate of fission and heat production, spent fuel rods
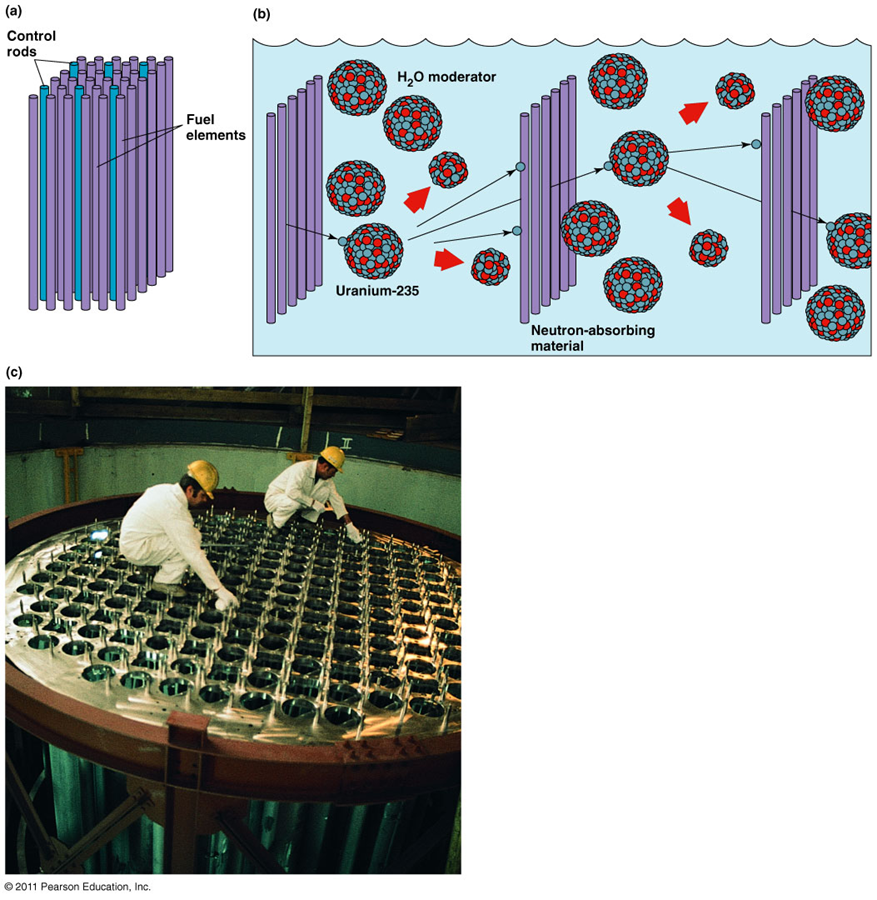
Control rods
•neutron-absorbing material inserted between the fuel elements
•Control the chain reaction in the reactor core
•Withdrawing and inserting starts and controls the chain reaction
•The fuel rods and moderator become intensely hot
Pressurized water reactor
•high-pressure water circulates through the reactor without boiling
•The superheated water then circulates through a heat exchanger and boils other, unpressurized water
•Isolating hazardous reactor materials
•If a reactor cracks, there would be a sudden loss of water from around the reactor
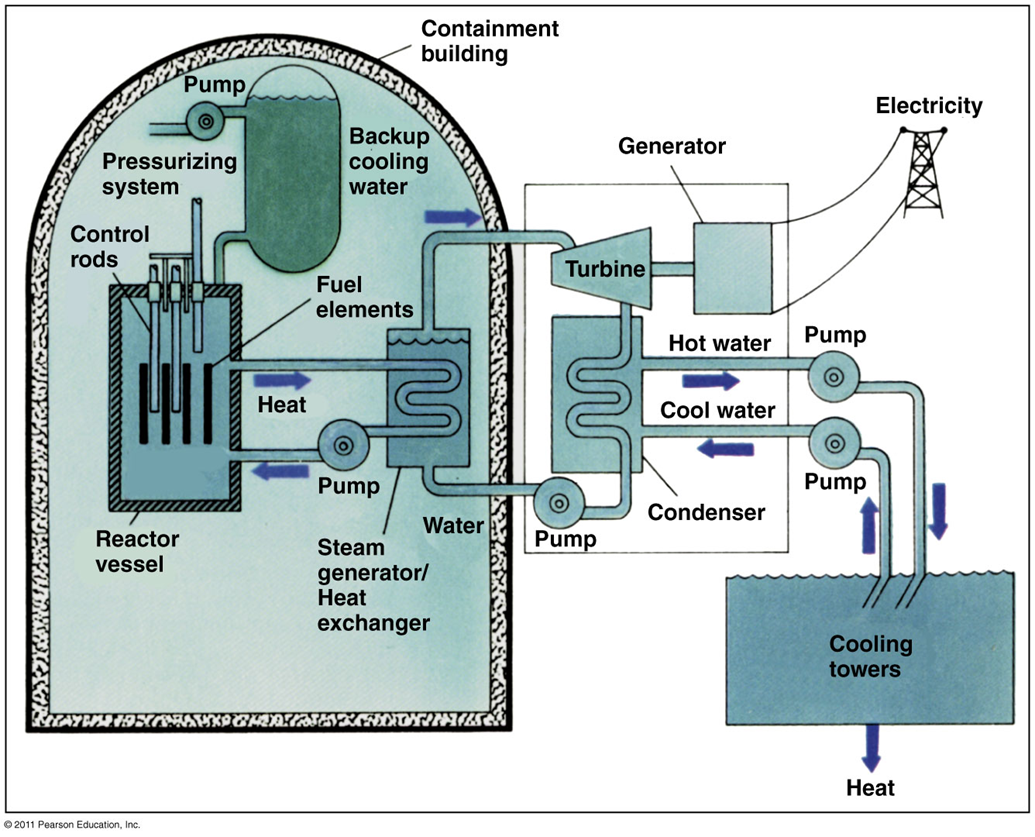
Boiling water reactor
•: water circulates through the reactor
•If a reactor cracks, there would be a sudden loss of water from around the reactor
Loss of cooling accident
LOCA
•occurs when a cracked reactor loses water
•The missing moderator would stop fission
•The fuel core could still overheat
•Backup cooling systems keep the reactor in water
•The entire assembly is encased in a concrete containment building
Meltdown
•enough energy is released to melt the core
•Molten material falling into remaining water could cause a steam explosion
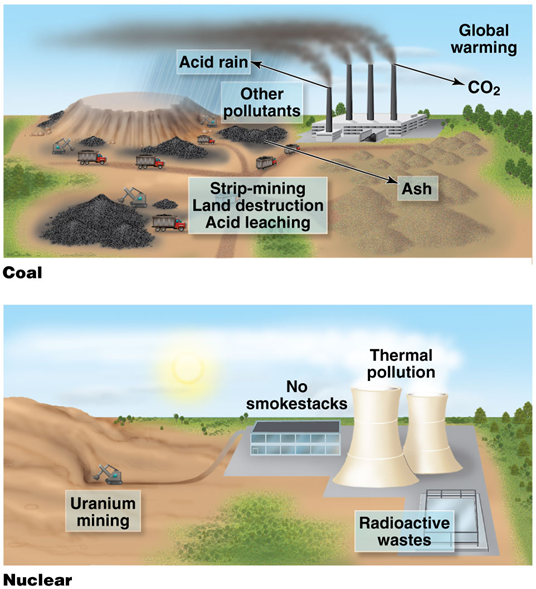
1000 MW nuclear plant
•Uses 30 tons of uranium
•Coming from 75,000 tons of ore
•Energy from fission of 1 lb of uranium equals 50 tons of coal
•Does not emit CO2 when operating
•Produces no acid-forming pollutants or particulates
•Low levels of waste gas
•250 tons of radioactive wastes require storage and disposal
Accidents can range from minor to catastrophic
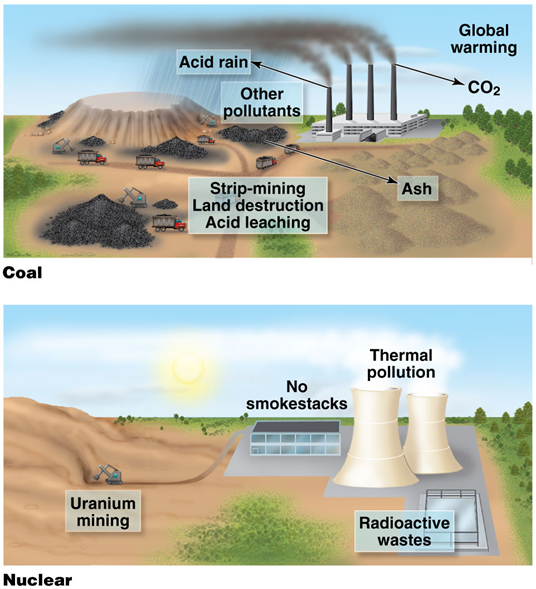
1000 MW coal plant
•Uses 2–3 million tons of coal
•Stripmining causes environmental damage, acid leaching
•Deep mining causes deaths and harm to health
•Emits 7 million tons of CO2
•Emits 300,000 tons SO2, particulates, other pollution
•Releases 100 times more radioactivity than a nuclear plant
•Produces 600,000 tons of ash
•Accidents could kill people or lead to fires
Radioactive emissions
fission of uranium or any other material creates new atoms
Radioisotopes
unstable direct products of fission
become stable by ejecting subatomic particles (alpha and beta particles, neutrons) Or high-energy radiation (gamma and X-rays)
Curie
•a measure of radioactivity
•1 gram of pure radium-226 is equivalent to 1Curie (1Ci = 3.7 x 1010 disintegrations per sec) also (1 Becquerel = 1 disintegration per sec)
Indirect products of fission
materials in and around the reactor can become radioactive by absorbing neutrons
High level radioactive waste
•direct products of fission
•Are highly radioactive
Low level radioactive waste
•indirect products of fission
•Are much less radioactive
•Include material from hospitals and industry
Strontium and cesium
Long-lived radioactive Isotopes from Nuclear Fission that is absorbed by the body and radioactivity in the bloodstream
Ionizing radiation
displaces electrons from tissues
•Leaves behind charged particles (ions)
•Breaks chemical bonds or changes molecular structures
•Is not felt or seen, but impairs molecular functions
Biological impact of radioactivity
Radioactive emissions can penetrate biological tissue
•Results in radiation exposure
•Absorbed dose: measures the exposure in
•Gray (GY): joules/kg (J/kg)
•Sievert (Sv): a weighted biological dose based on the type of radiation (also J/Kg)
•Rem: an old term; equals 0.01 Sv
High radiation dose
radiation can prevent cell division
•Radiation sickness: exposure (> 1 Sv) prevents replacement or repair of blood, skin, other tissues
•Can lead to death in days or weeks
Radiation sickness
exposure (> 1 Sv) prevents replacement or repair of blood, skin, other tissues
Low radiation dose
•can damage DNA
•Cells can form tumors or leukemia
•Damaged eggs or sperm can cause birth defects
•Effects of exposure may go unseen for 10–50 years (Carcinogenesis)
•Other effects: weakened immune system, mental retardation, cataracts
Radiation exposure
•Health effects are directly related to the level of exposure
•Doses between 100 and 500 mSv increase the risk of developing cancer
•The National Research Council (NRC) found no safe level of radiation
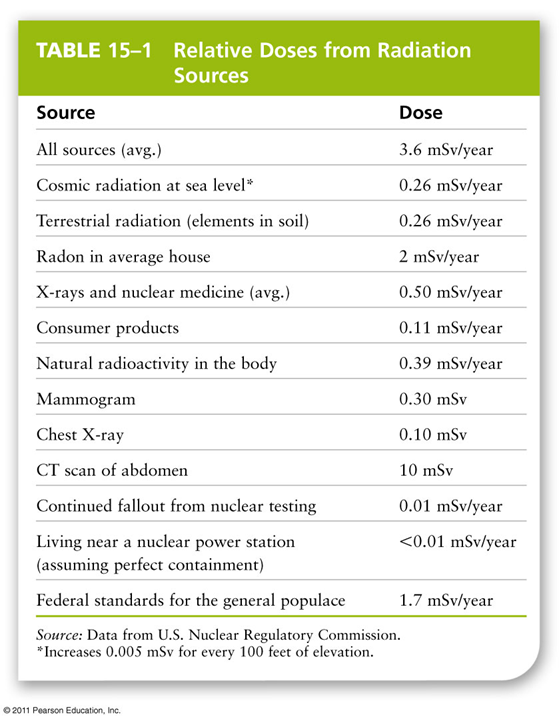
Radiation sources
•Normal background sources: uranium and radon gas from Earth’s crust
•Cosmic rays from outer space
•Medical and dental exposure: X-rays, CT scans
Radioactive decay
•unstable isotopes become stable by ejecting particles and radiation
•Is harmless as long as it is kept away from organisms
Radioactive reprocessing
•most 235U and 239Pu is recovered and recycled
Until recently, it was forbidden in the U.S
Short term radioactive waste containment
allows radioactive decay of short-lived isotopes
•In 10 years, fission wastes lose 97% of radioactivity
Long term radioactive containment
•the EPA recommends a 10,000 year minimum
•Congress extended the protection to 1 million years
Tanks and casks
•Spent fuel is stored in deep swimming-pool like tanks
•Short-term containment
•Prevents escape of radiation
•Can hold 10–20 years of spent fuel
•Pools were 50% filled in 2004 and will be 100% filled by 2015
•Air-cooled dry casks hold spent fuel for the short term
•Engineered to withstand floods, tornadoes, etc.
Military failures with radioactivity
•Some of the worst failures in handling radioactive waste occurred at U.S. and former Soviet Union military facilities
•The U.S. nuclear program killed 4,000 and sickened 36,500 people
•Leaking liquid wastes have contaminated water, wildlife, sediments, and groundwater
•Activities have been shrouded in secrecy
•The DOE has already spent $50 billion in cleanup
•The final cost could be $250 billion
Lake Karachay, Russia
•For 20 years, nuclear wastes were discharged from Chelyabinsk-65 (Russian military weapons facility) into the Techa River and Lake Karachay
•In the 1950s-60s, standing for 1 hour on the shore of Lake Karachay led to death within a week
•In 1967, the lake dried up and the radioactive dust spread and exposed hundreds of thousands of people
•This is the most polluted lake on Earth
•Authorities filled the lake with concrete, rocks, soil
Post WWII nuclear program
•The end of the Cold War caused the U.S. and former Soviet Union to dismantle nuclear weapons
•Plutonium weapon reactors were closed
•Money helped Russia destroy missile silos
•Nuclear submarines and bombs were dismantled
•Megatons to Megawatts program: a U.S. company diluted weapons-grade uranium to power-plant uranium
•This uranium is sold to U.S. power plants
•The Russian government received $5.7 billion
•Half of U.S. power plant uranium comes from this program
Nuclear geological burial
•the safest option for disposing of highly radioactive spent fuel
•No nation has buried the fuel
•Many nations haven’t even found burial sites
•Selected sites have questionable safety
•We can’t guarantee a stable rock formation for tens of thousands of years
•Possible volcanoes, earthquakes, groundwater leaching
•Radioactive wastes could escape by these events
Nuclear waste policy act, 1982
in 1998, the federal government must start receiving nuclear waste from commercial power plants.
Congress selected Yucca Mountain, Nevada as the nuclear waste site for the U.S.
Reason: NIMBY (not in my backyard) syndrome has prevented location of a long-term containment site in the U.S.
States prohibit nuclear waste disposal
Yucca mountain
Congress selected Yucca Mountain, Nevada as the nuclear waste site for the U.S.
In 1989, Nevada prohibited nuclear waste storage
•The U.S. government overrode the prohibition
•$10 billion has been spent over the last 25 years studying Yucca Mountain
•In 2008, the D O E submitted a license application
•The N R C recommended approval
•Critics questioned if safety could be guaranteed
•The Obama administration stopped the Yucca program for health and safety concerns
•A Blue Ribbon Commission explored other options

Nuclear power accidents
•Rancho Seco Nuclear Power Plant, California (1978)
•Human error, near meltdown, no radioactivity released
•Three Mile Island Nuclear Power Plant, Pennsylvania (1979)
•Faulty instruments / human error, 10 MCi released
•Chernobyl, Russian (1986)
•Human error, meltdown, 100-200 MCi released
•Kashiwazaki-Kariwa Nuclear Power Plant, Japan (2007)
•Earthquake of July 16, 2007, negligible release (~10mCi)
•Fukushima Dalichi Nuclear Power Plant, Japan (2011)
•Earthquake & tsunami of March 11, 2011
Near meltdown, but major leak: released >24 MCi
Three mile island
•In Pennsylvania, on March 28, 1979, Nuclear Power Plant suffered a partial meltdown
•A lack of power shut down the steam generator
•An open valve drained water from the reactor vessel
•Operators shut down the emergency cooling system
•Gauges incorrectly reported the reactor had water
•The uncovered core suffered a partial meltdown
•Released 10 million curies of radioactive gas
•No injuries or deaths have been reported
•The badly damaged reactor (reactor #2) will not be reopened; reactor #1 is still operating
Chernobyl
•On April 26, 1986, power plant had a meltdown in one of the reactors
*This disaster killed 31 and put thousands of others at risk of future cancer death
•To test generators
•Engineers disabled the safety systems
•Withdrew control rods, shut off steam to generators, and reduced coolant water to reactor
•Lacking coolant, radioactive heat energy increased
•Too late, engineers inserted control rods
•Explosions blew off the top of the reactor
•Fire burned for days
Radioactivity spread over thousands of miles

Consequences of Chernobyl
•135,000 people were evacuated
•The reactor was sealed in concrete and steel
•A barbed-wire fence surrounds the 1,000-square-mile zone
•Soil and all vegetation is contaminated
•Two engineers were killed by the explosion
•28 died within months from radiation
•Children have thyroid cancer from contaminated milk
•20–70 times higher than normal rates of thyroid cancer seen
•The shelter over the plant is decaying
•A $1.4 billion new containment structure will take 5 years to build
•It will be large enough to house the Statue of Liberty
•The environmental effects of the disaster are complex
•Immediately after the disaster, plants and animals died
•Including an entire forest of pine trees
•Later, trees tolerant of radiation grew
•Animals returned
•They remain healthy, even when exposed to radiation and they experience mutations
•The evacuation of humans has allowed elk, deer, bear, and birds to thrive
Including the rare Przewalski’s horse

Fukushima Daiichi
•There was no way to predict or protect the power plant from an earthquake and tsunami
•Power loss from the grid and backup generators left only eight hours of batteries for cooling the reactors
•Without water in the pools, the fuel rods could not be cooled
•Dangerous gases could not be vented from buildings
•The Union of Concerned Scientists thinks it could happen in the United States—but the N R C disagrees
These accidents were due to human error or natural disasters, causing a loss of public trust in nuclear safety
Modern nuclear safety
•The NRC has upgraded safety standards
•Design, maintenance, training
•Nuclear plants are safer than ever
•There is no such thing as an inherently safe reactor
•Plants have active safety features: operator-controlled
•Along with external power and electrical systems
•New plants rely on passive safety: devices and structures
•Impossible for the reactor to go beyond acceptable power levels, temperature, and emissions
Terrorism and nuclear power
•A jetliner could not penetrate the containment center’s thick walls
•But it could destroy the control building and bring on a LOCA
•Terrorists could attack the plant, overcome the guards, and manipulate the controls to cause a core meltdown
•Many of the NRC’s mock terrorist attacks have penetrated plant security
P/C Breeder reactors
Pros | Cons |
Safety and security precautions are greater The half-life of 239Pu is 24,000 years | Reactors are more expensive to build and operate |
Large amounts of 239Pu could cause serious meltdowns | Extract more energy from recycled nuclear fuel |
239Pu can be made into nuclear weapons more easily | They produce much less high-level waste than conventional power plants |
Fusion reactor
•aims to carry out controlled fusion
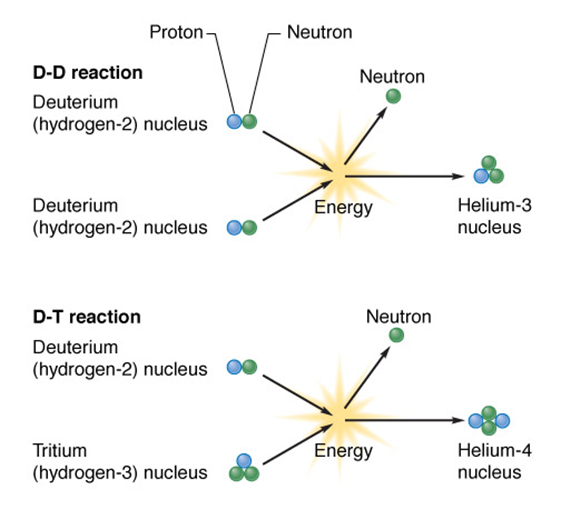
•In theory, fusion can provide pollution-free energy using deuterium and tritium
•Deuterium (2H): naturally occurring, nonradioactive
•Extracted from seawater
•Tritium (3H): radioactive isotope artificially produced
Fusion energy
•Fusion power consumes energy
•It needs extremely high temperature (3 million degrees C) and pressure to fuse 2H + 3H
•A hydrogen bomb gets the temperature and pressure by using a fission bomb as an igniter
•Fusion reactors need effective, costly designs to prevent leaking radioactive tritium
•The break-even point has not been reached
Tokamak
ionized H is held in a magnetic field
•Containing the hydrogen while it is being heated millions of degrees is a major technical problem
•No known material can withstand this heat - tokamak combats this
Laser fusion
a tiny pellet of frozen H is dropped in target and laser beams heat it to the point of fusion
Z machine
powerful pulses of electricity heat the hydrogen
International thermonuclear experimental reactor
ITER
•A prototype fusion reactor of the Tokamak type
•Being built in France, costing over $20 billion
•To produce 10 times as much energy as it uses
Nuclear energy future
•Nuclear power is expensive, faces opposition, and depends on government subsidies
•However, 65 reactors are under construction
•437 reactors currently generate 13.5% of electricity
•Many nations are going ahead with more plants
•Russia, Finland, the United Kingdom
•Asian countries (China, South Korea, India)
•The Fukushima disaster will slow, not stop, nuclear energy
•Nuclear power will double by 2030