Old Exam Review Flashcards
1/167
Earn XP
Description and Tags
A comprehensive set of flashcards based on the old exam questions and lecture materials covering organic chemistry concepts.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
168 Terms
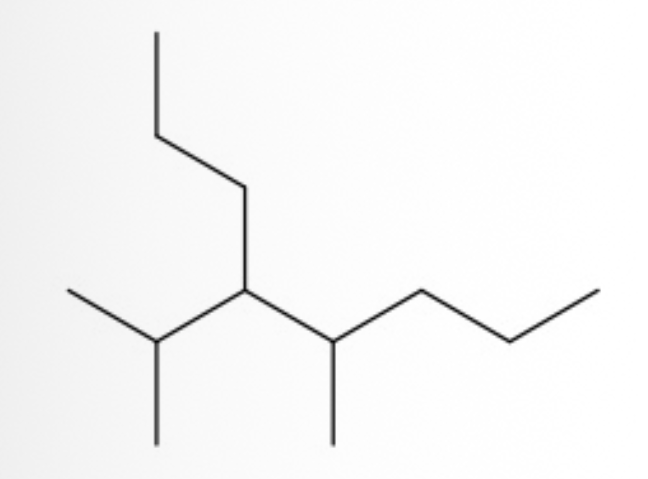
What is the IUPAC name ?
4-isopropyl-5-methyloctane.
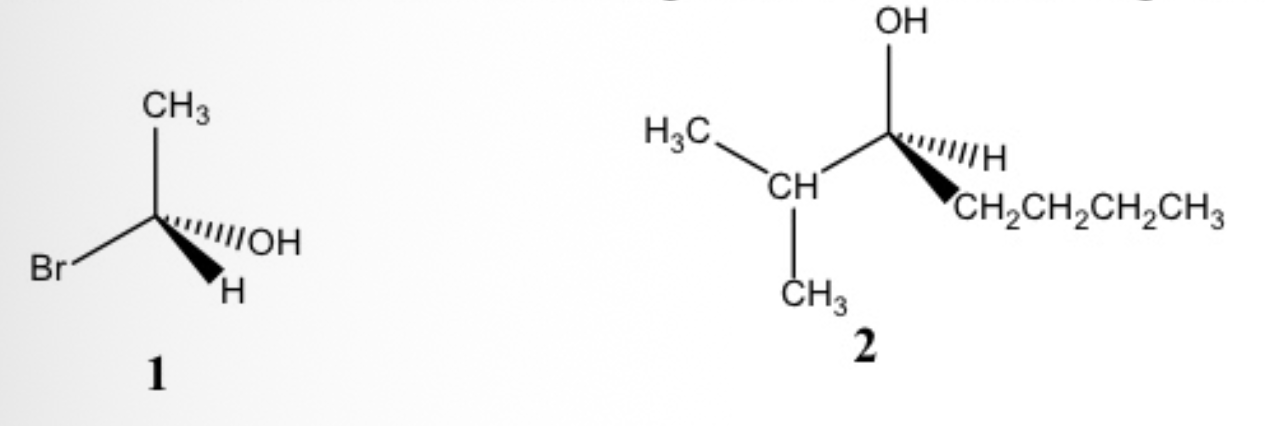
Which of the following has/have the S configuration: 1 or 2?
Only 2
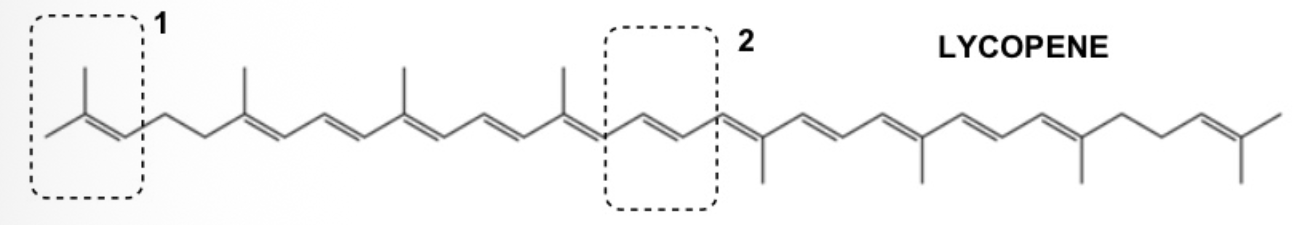
Lycopene (below) is a bright red antioxidant carotenoid hydrocarbon found in tomatoes as well as other red fruits. Question: Select the correct E/Z/neither stereochemistry assignment for the C=C bonds labelled 1 and 2.
1= neither E or Z; 2=E
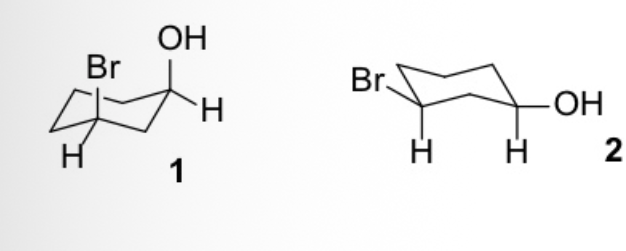
select the correct definition for 1 and 2
They are two conformers of the same molecule
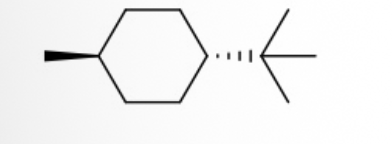
Identify the IUPAC name of the compound below
Trans-1-(tert-butyl)-4-methylcyclohexane
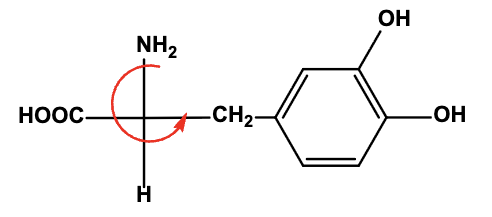
Select the TRUE statement about the following structure representing the chemical Dopa.
This is S-Dopa (the anti-Parkinson medication).
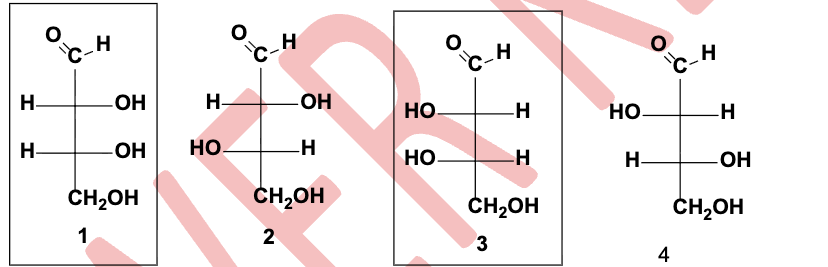
How are the carbohydrates in the box related?
They are enantiomers
Select the molecule that has a molecular dipole moment.
NH3
What is the approximate value of the C-C-C bond angle in CH3-C=CH?
180°
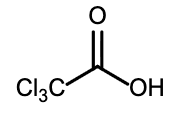
Select the strongest acid
Select the molecule that has NO internal plane of symmetry (which one is chiral)
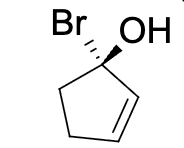
Which molecule can have cis trans isomerism?
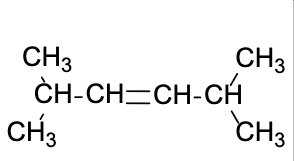
Which one of the following structures represents a different stereoisomer from the other three?
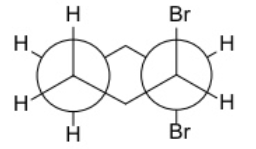
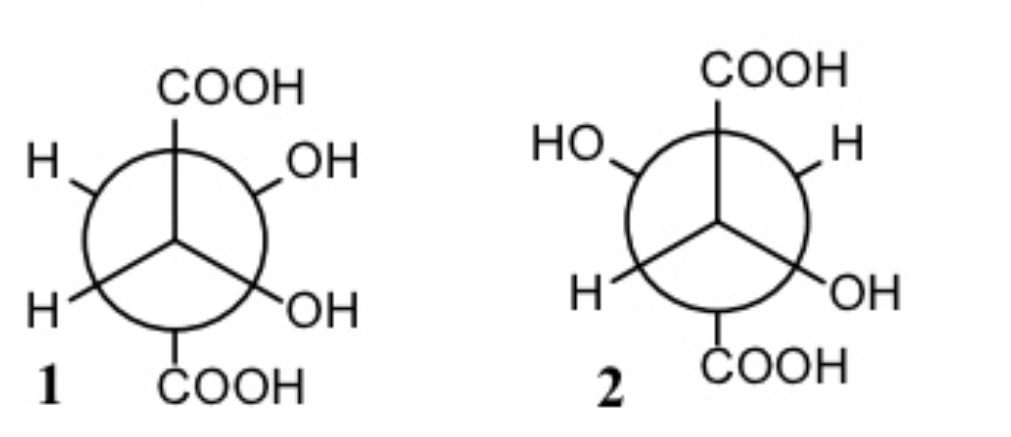
Following are two Newman projections for tartaric acid, an organic acid that occurs naturally in grapes and fruits. What is the relationship between (1) and (2)?
They are diastereomers.
Which of the following compounds has a pKa ~ 16?
CH3CH2-OH
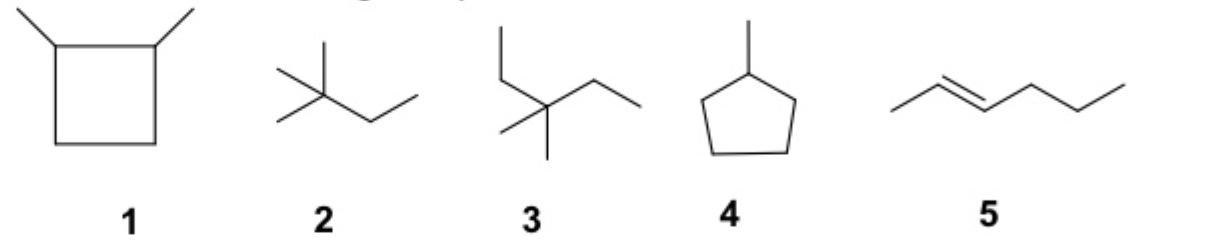
Which of the following compounds are constitutional isomers?
1,4, and 5
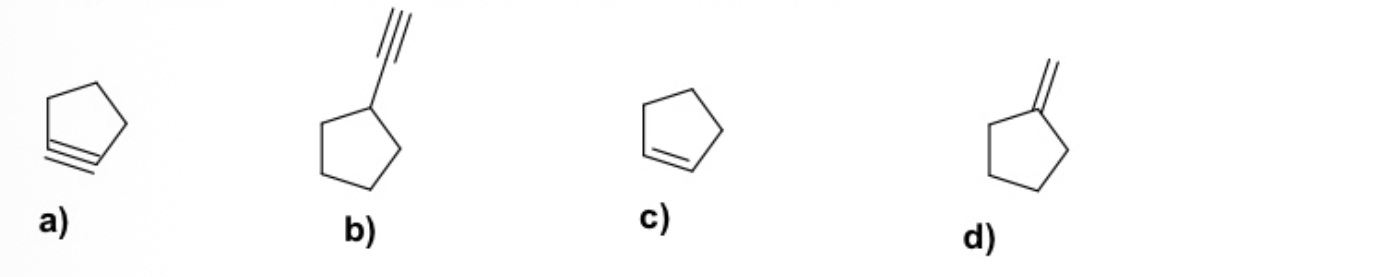
What molecule is most unstable and cannot be made?
A

Select the TRUE explanation for the following observation:
The sp3 hybridized carbons in cyclopropane have a bond angle of 60°, much smaller than the expected ideal bond angle of 109.5° observed in cyclohexane, causing an increase in the potential energy for cyclopropane.
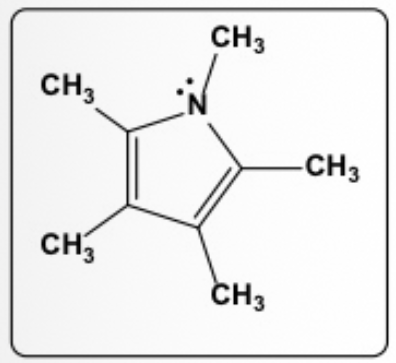
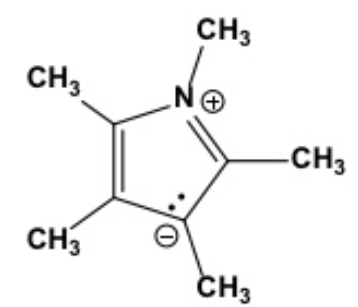
Select the only contributing and meaningful resonance structure of the compound below at left
Which of the following is the definition of a pair of diastereomers?
A pair of stereoisomers that are not mirror images of one another
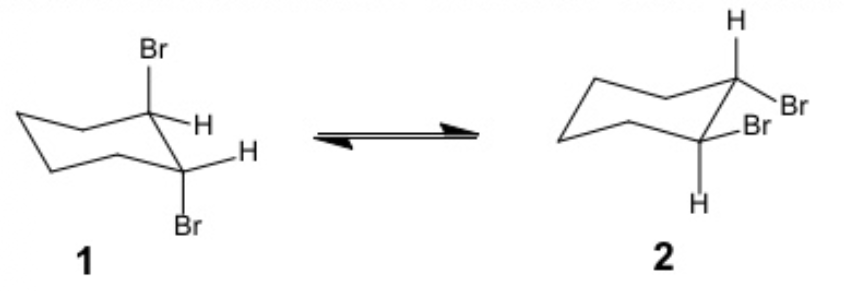
These are two conformers of the same molecule. Select the TRUE statement
Conformer 2 is more stable than Conformer 1
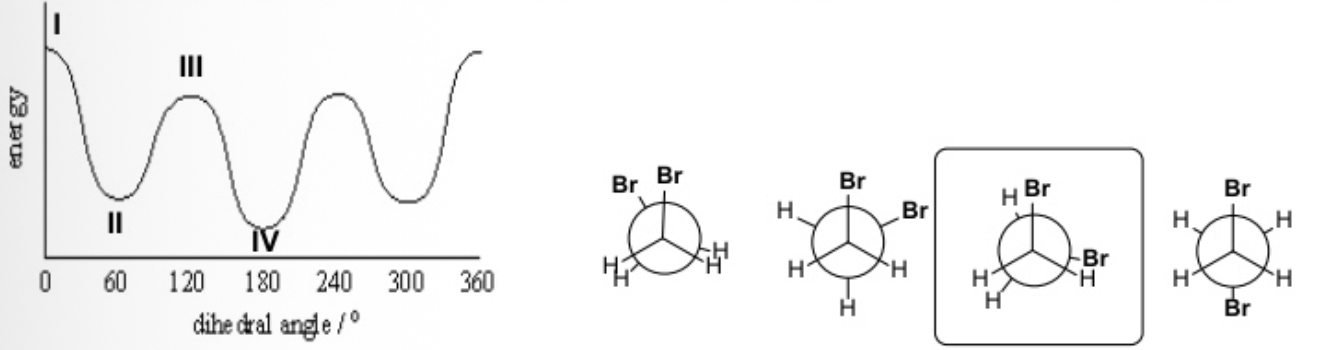
Matching the conformation in the box to the correct point in this energy diagram.
The conformer in the box matches III
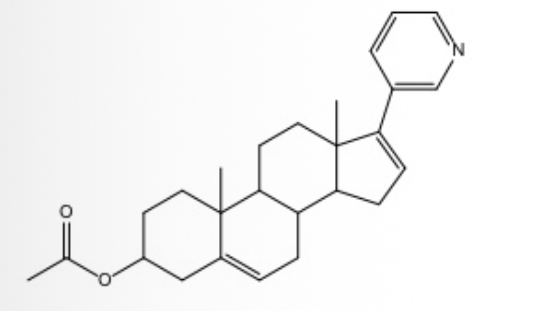
Identify the number of chiral centers in the given molecule.
six
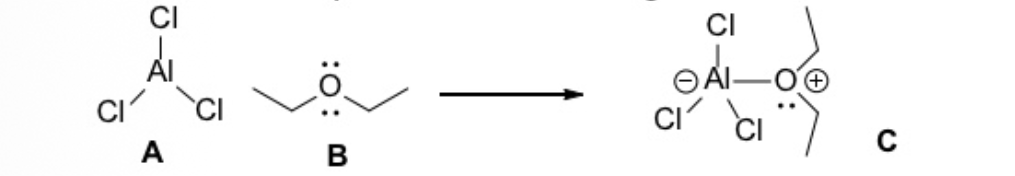
Identify the correct Acid-Base description for the following reaction.
A is the Lewis Acid; B is the Lewis base
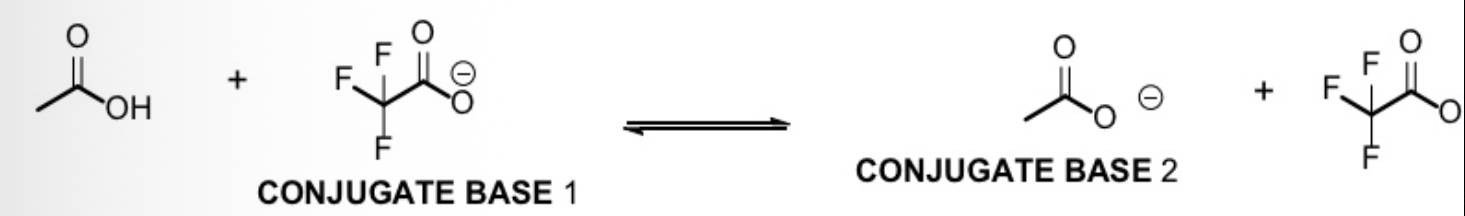
Indicate in which direction the equilibrium lies for the following acid-base reaction
Left, because the conjugate base 1 on the left is more stable
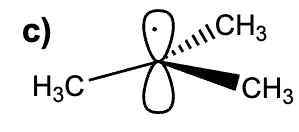
Select the accurate orbital representation of the tert-butyl radical.
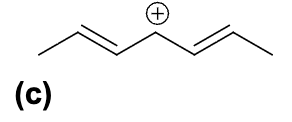
Which of the following carbocations is the most stable ?
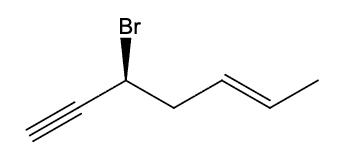
What is the IUPAC name of the following compound?
(S,E)-3-bromohept-5-en-1-yne
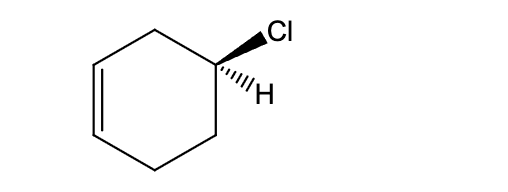
Identify the correct hydrogen bonding interaction.
(S)-4-chlorocyclohex-1-ene
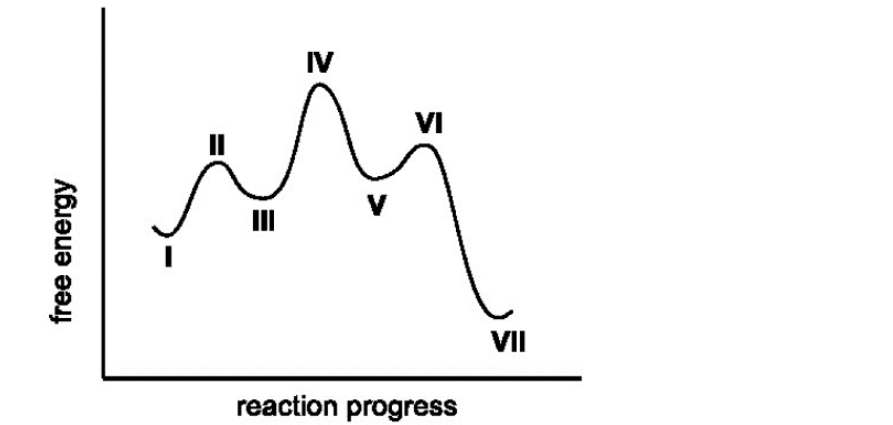
5. Select the TRUE statement regarding the coordinate energy diagram for the reaction 1→ VII.
V is the least stable intermediate
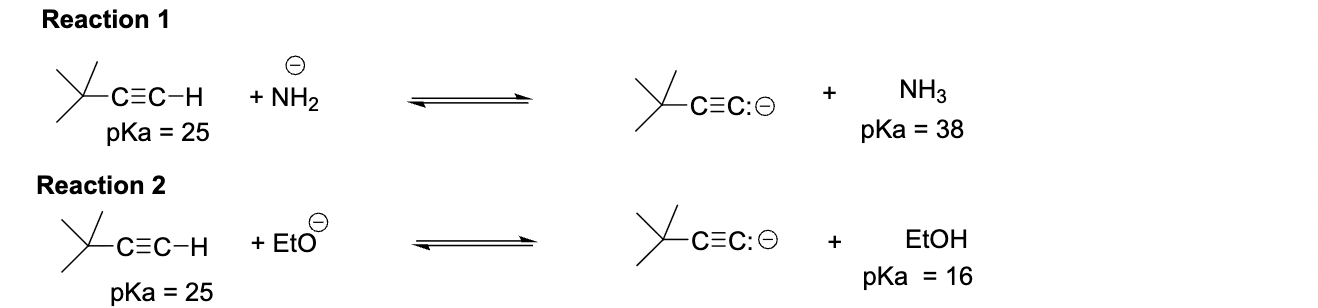
Acetylides anions are very useful reagents for C-C bond formation reactions. Which reaction conditions would you choose to prepare the following acetylide anion quantitatively?
Reaction 1
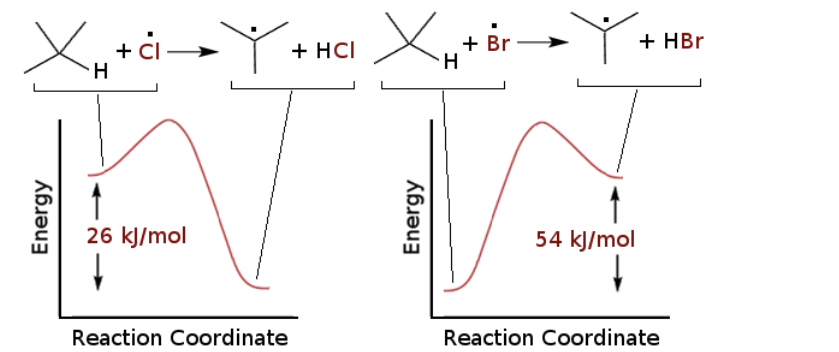
Consider the energy diagrams for the H-abstraction step in the halogenation reaction with Br radical (Br) and Cl radical (Cl) and select the TRUE statement
H-abstraction by Br is more regioselective because it is endothermic and the late T-state has more radical character (product-like)
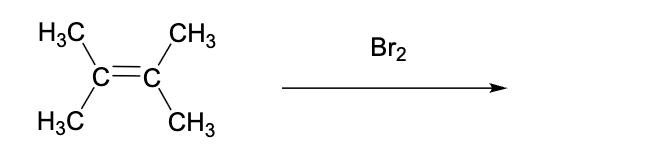
This bromination reaction will produce …. (select the TRUE answer to complete the sentence)
a product that is not chiral
Which of the following reagents adds with SYN stereochemistry to alkenes?
OsO4
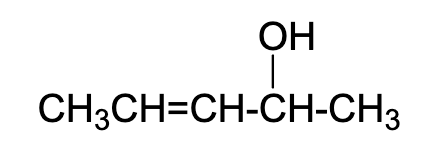
What is the number of possible stereoisomers in this molecule? careful
4
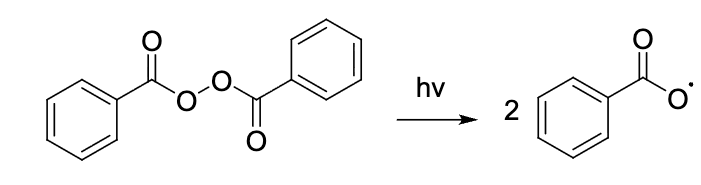
Identify this step.
b) Radical propagationRadical initiation
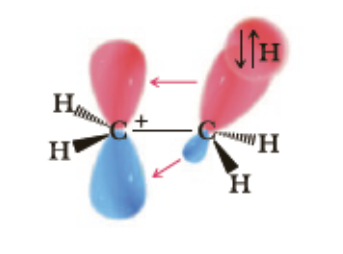
This cartoon diagram represents.... select the TRUE answer to complete the sentence.
the hyperconjugation effect in the ethyl carbocation
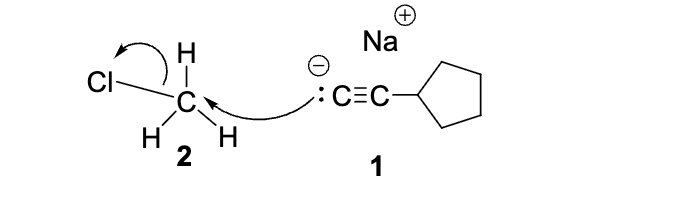
Select the TRUE statement regarding this step:
1 is the Nucleophile and 2 is the Electrophile
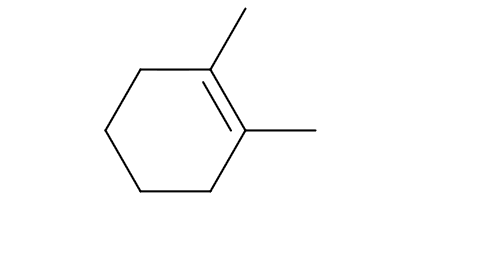
How many allylic positions (not allylic Hydrogens… allylic POSITIONS) can you find in this molecule?
4
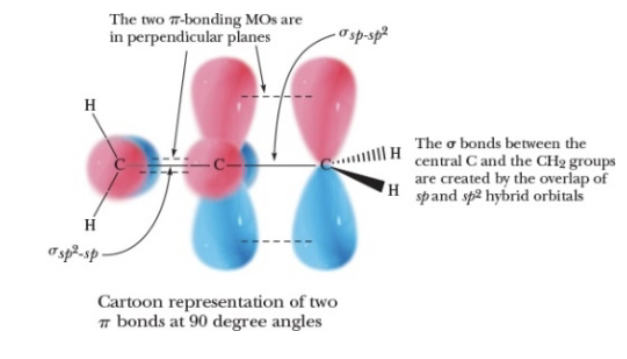
The following cartoon is the representation of…. Complete with the TRUE definition
an allene
Select the TRUE explanation for the Markovnikov regioselectivity of the H+ catalyzed hydration of alkenes
The relative stability of the carbocation intermediates
Which of the following bonds has the highest bond dissociation enthalpy?
C-F
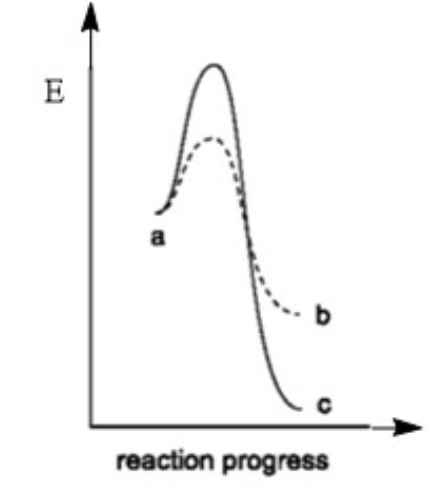
Select the FALSE statement regarding the reaction coordinate diagrams for two separate reactions: a→b (dashed line ---- ) and a→c (solid line ) shown below
Reaction a→b is the catalyzed version of reaction a→с
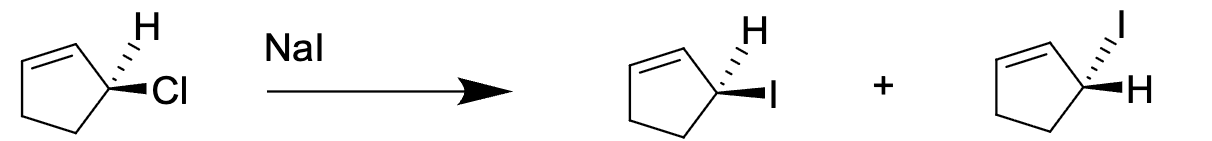
Select the TRUE explanation for this result
The reaction follows a SN1 mechanism because it can form a very stable allyl cation
The reaction of 1-bromopropane with sodium azide (NaN3) in acetone gives 1-azidopropane. What is the effect of doubling the concentration of NaN3 on the rate of the reaction? (Hint: what mechanism do you
expect?)
The rate increases by a factor of 2
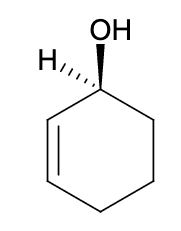
What is the IUPAC name of the following compound?
(1S)-cyclohex-2-en-1-ol
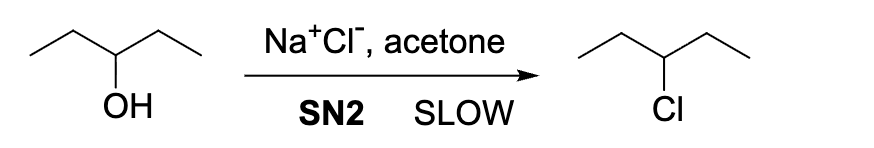
The SN2 pathway for the reaction below has a very low rate. why?
OH- is a poor leaving group
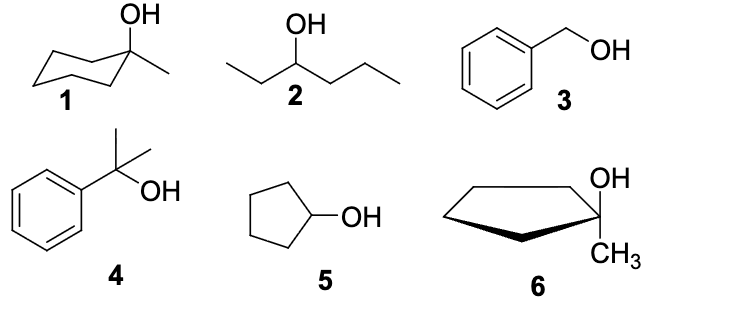
Which of the following alcohols will NOT be oxidized by Jones reagent (H2SO4, CrO3)
1,4,6
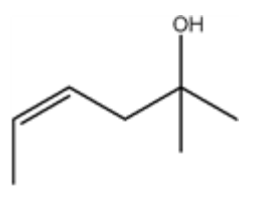
What is the IUPAC name of the following compound?
(Z)-2-methyl-4-hexen-2-ol
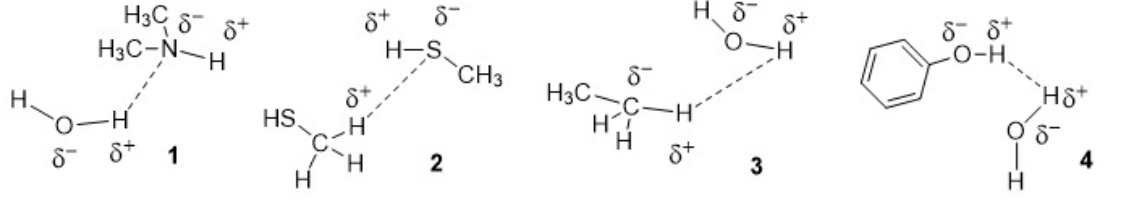
Which one (s) of the following is(are) the correct representation of a Hydrogen-Bonding interaction?
1 only
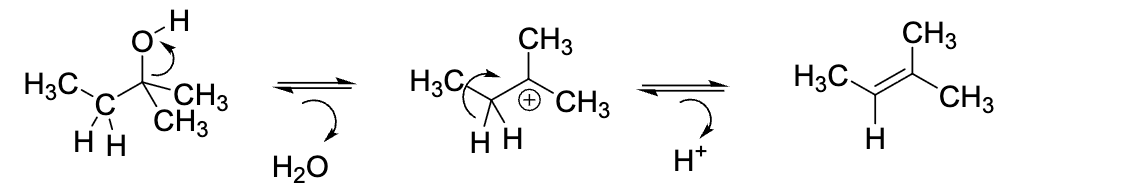
What is wrong with this mechanism of an acid-catalyzed dehydration reaction?
First the OH must be protonated by H+ to become a good leaving group
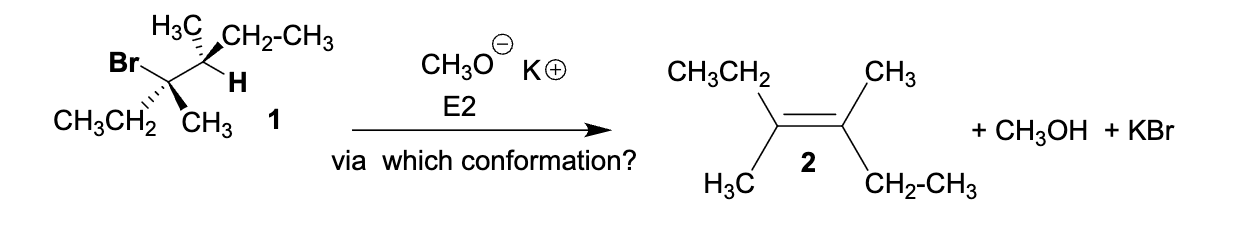
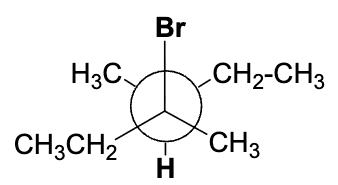
Which one of the following Newman projections will lead to the E2 elimination reaction of compound 1 to form 2 (E stereoisomer).
Select the FALSE statement related to a SN1 reaction mechanism.
If we start with an enantiomerically pure starting material the SN1 product has inverted configuration
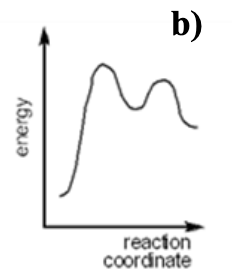
Which of the following energy diagrams represents an ENDOTHERMIC SN1 reaction

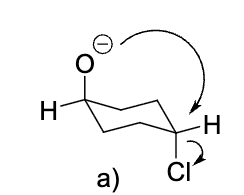
The bicyclic ether 1 below is formed from an intramolecular Williamson reaction (SN2). Select the correct
mechanism below.
Which of the following will be the BEST Nucleophile in polar aprotic solvent
CH3O-
Which of the following is the STRONGEST Base? (pKa of the conjugated acid is provided to help you!)
pka =25
CH3CCH
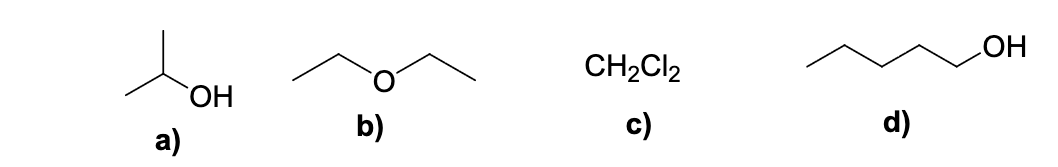
Which of the following compounds has the highest solubility in water ?
a
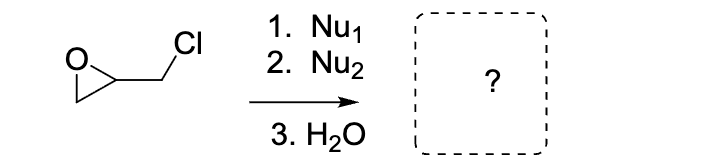
c
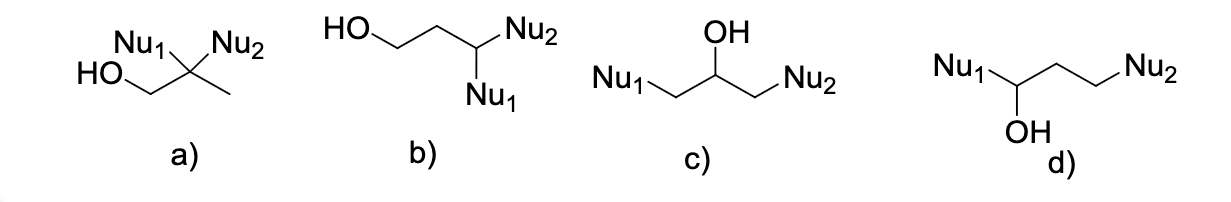
![<p>Select the sequence of reagents needed to accomplish the following reaction [TBAF = Bu4NF]</p>](https://knowt-user-attachments.s3.amazonaws.com/4c173fc4-f6d8-498a-8c6d-d9fc6e5db2e5.png)
Select the sequence of reagents needed to accomplish the following reaction [TBAF = Bu4NF]
1) Me3SiCl, Et3N 2) NaNH2 3) D2O 4) TBAF
Select the TRUE statement regarding the type of reaction shown below
it is a reduction
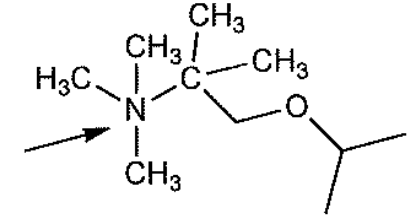
Select the correct formal charge on the atom indicated by the arrow
+1
Which of the following is the STRONGEST Base? hint: pKa of the conjugated acid is provided
pKa CH3CCH = 25
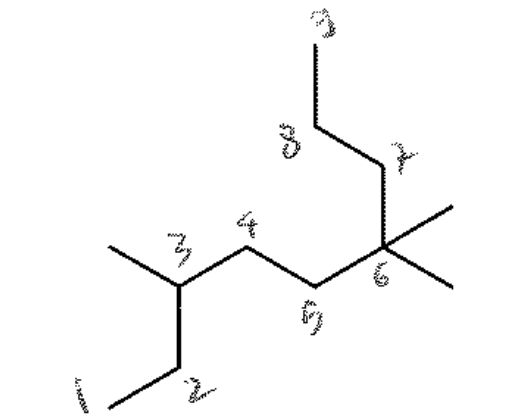
Select the correct IUPAC name for the following compound
3,6,6-trimethylnonane
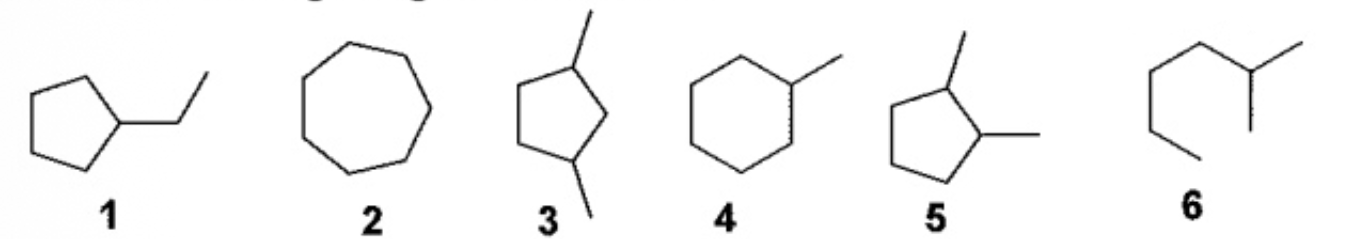
Answer the TRUE statement regarding these molecules
6 is the only molecule that is not a structural(constitutional) isomer of the others are
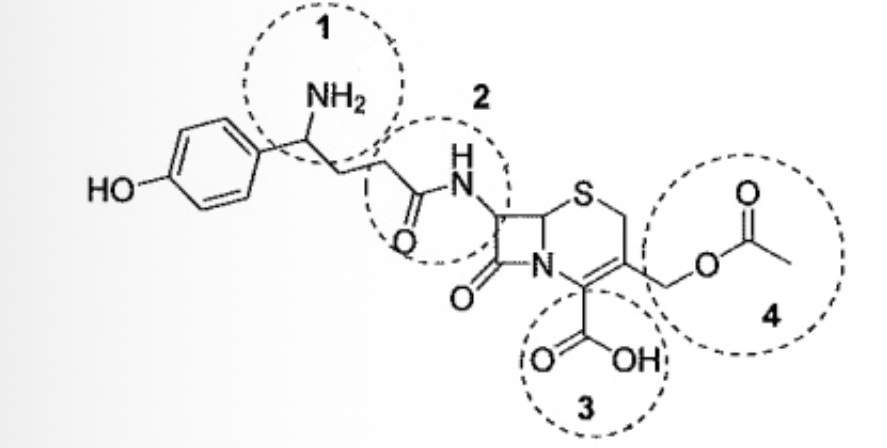
Cephalosporins are a class of antibiotics first discovered in 1945. Identify the circled functional groups in the cephalosporin below
1= amine; 2= amide; 3= carboxylic acid; 4 = ester
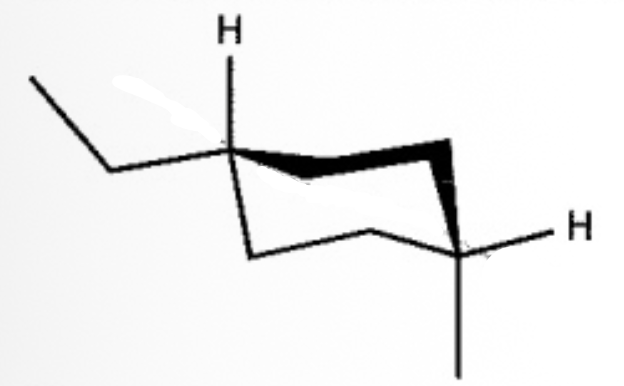
What is the IUPAC name of the following compound?
cis-1-ethyl-4-methylcyclohexane
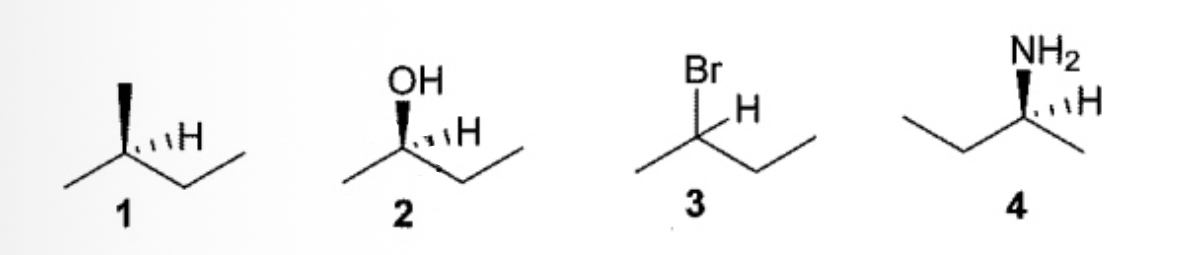
Which one(s) of the following compounds has(have) R configuration?
only 2
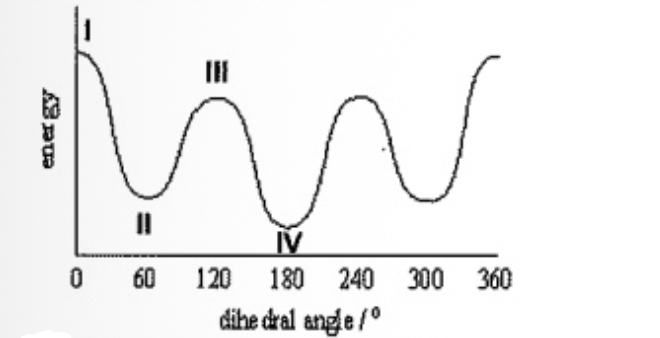
Select the correct assignment matching this energy diagram.
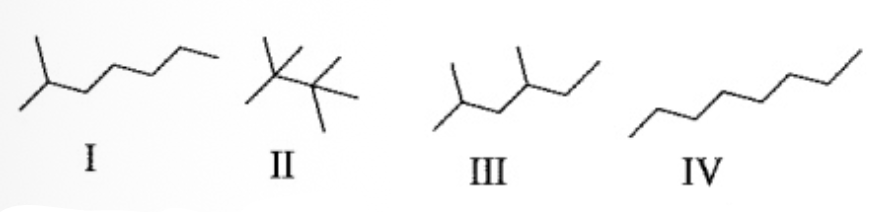
Rank the following alkanes in order of increasing boiling point (lowest b.p. < highest b.p.)
II<III<I<IV
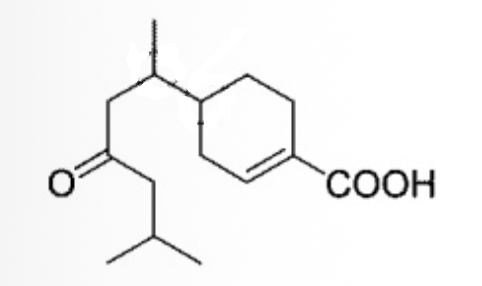
How many stereoisomers are possible for the following molecule?
4
Which of the following molecules are polar (meaning have a molecular dipole moment)?
(1) NH3 (2) BF3 (3) H2O (4) CCI4 (5) Cl2C=CCI2 (6) CH3CI
1,3 and 6
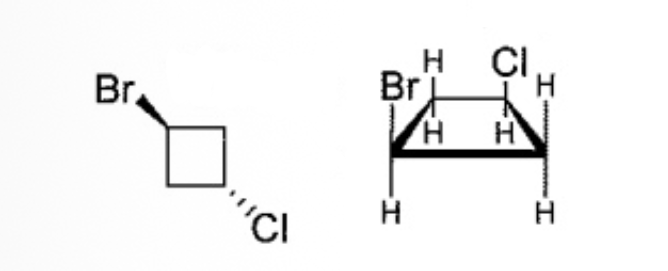
Select the correct relationship for this pair of molecules
Cis-trans Stereoisomers
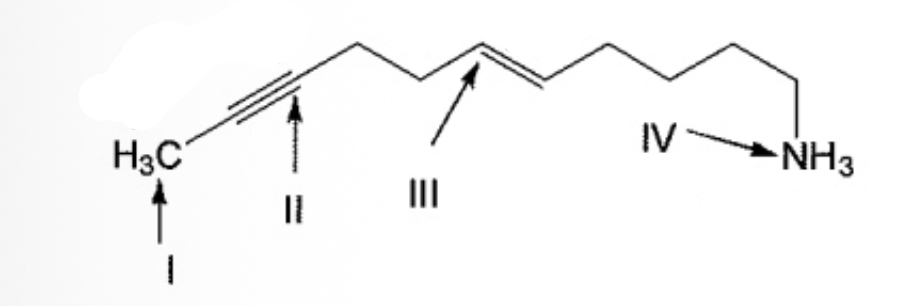
What is the correct order of hybridization state for the numbered atoms in the following compound?
| sp3; II sp; III sp2; IV sp3
Which of the following represents the molecular formula for a non-cyclic alkane?
C20H20
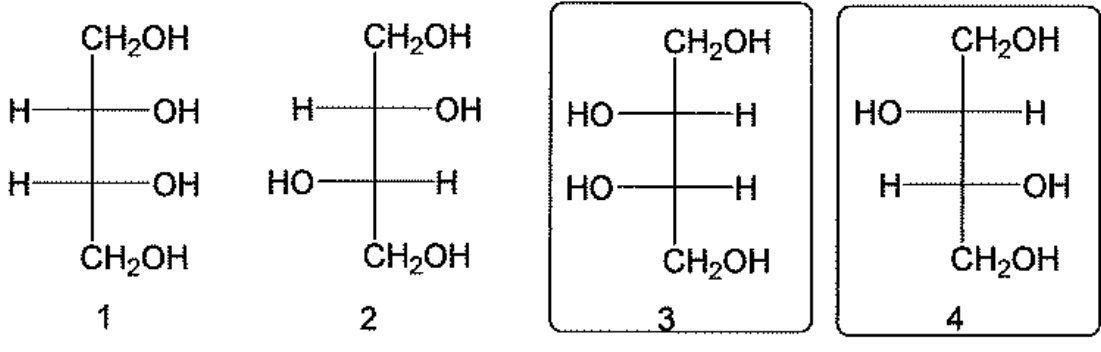
How are the carbohydrates selected in the boxes related?
They are diastereomers
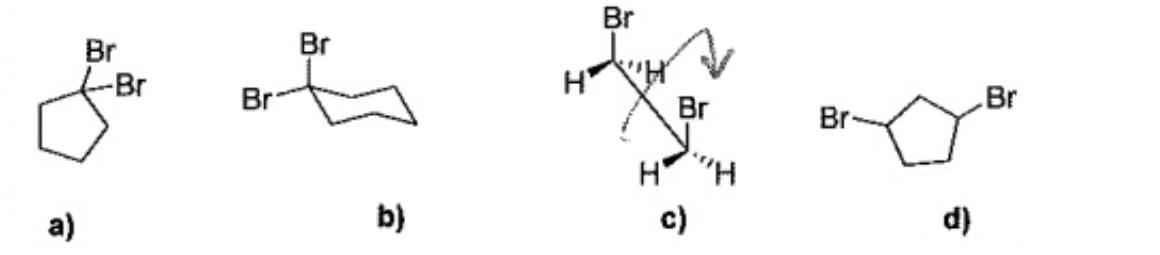
Which one of the following molecules can have cis trans isomerism?
D
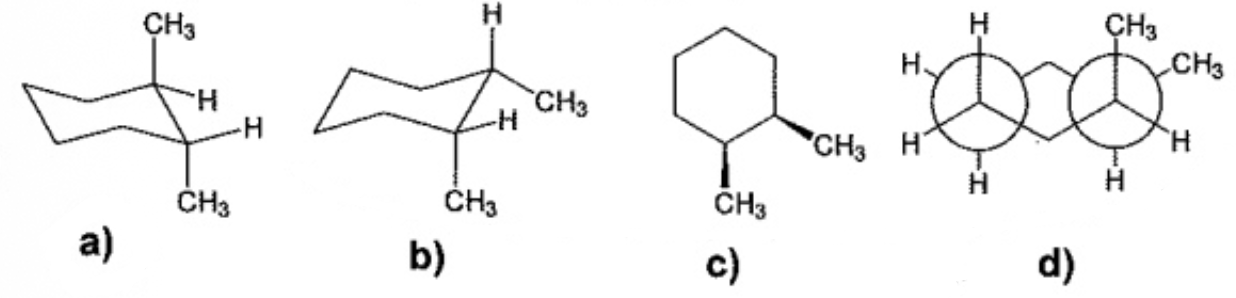
Which one of the follow.g structures represents a different stereoisomer from the other three?
A
What is the approximate value of the C-C-C bond angle in alkyne CH3-C=CH ?
180 degrees
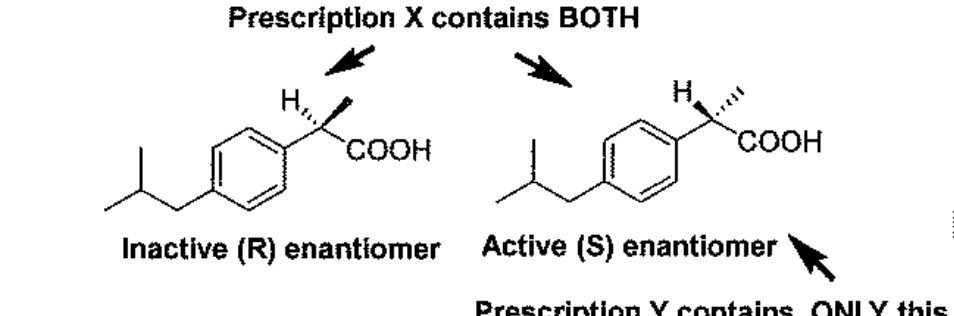
Prescription Y contains only the active enantiomer of ibuprofen; Prescription X is a racemic mixture of the active + inactive enantiomers of ibuprofen. Knowing this, which of the following statement is TRUE?
A 10 mg dose of Y will be more potent than a 10 mg dose of X
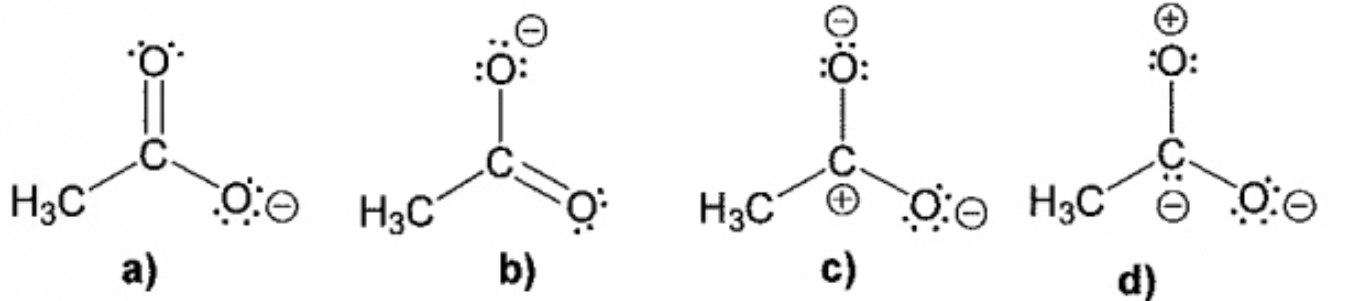
Which one is the LEAST important contributing form for the resonance structure of acetate ion
D
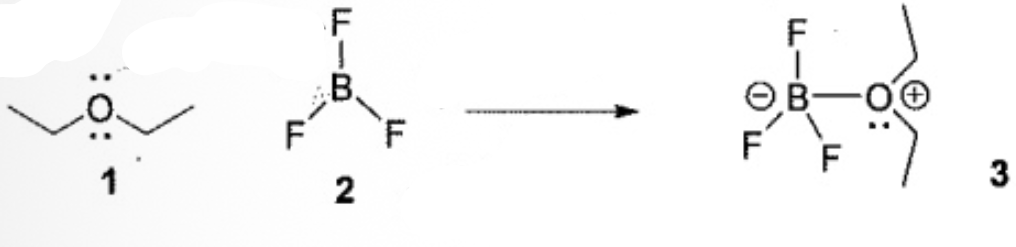
Identify the correct Acid-Base description for the following reaction
1 is the Lewis base; 2 is the Lewis acid
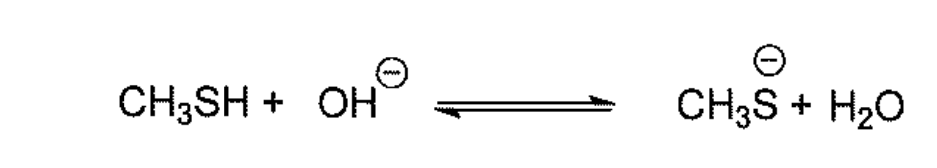
Select the correct statement for the following reaction
CH3SH is an acid
Which of the following statements regarding a pair of R and S enantiomers is FALSE?
The S enantiomer always rotates the plane of polarized light to the left and the R enantiomer always rotates the plane of polarized light to the right
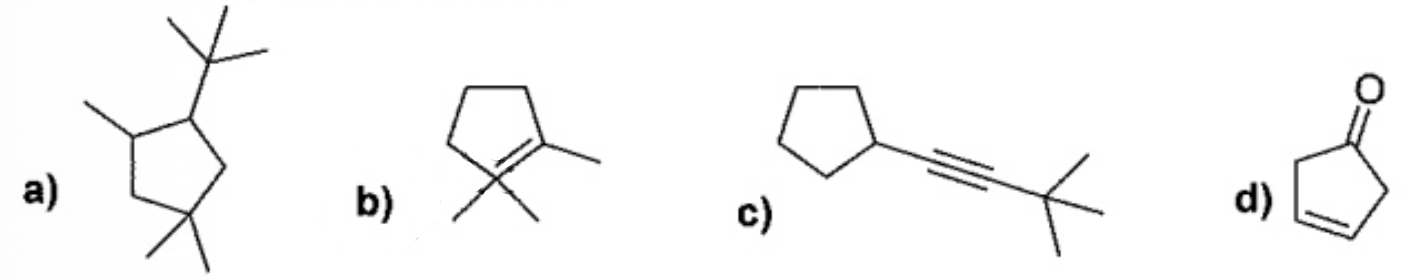
Which of the molecules below has a mistake?
B
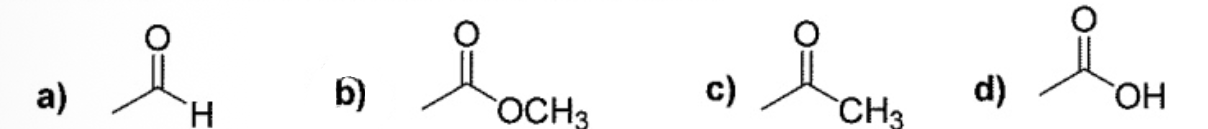
Esters have often pleasantly fragrant and are found in many natural and artificial flavoring and in perfumes. Question: which of the following functional groups is an ester?
B
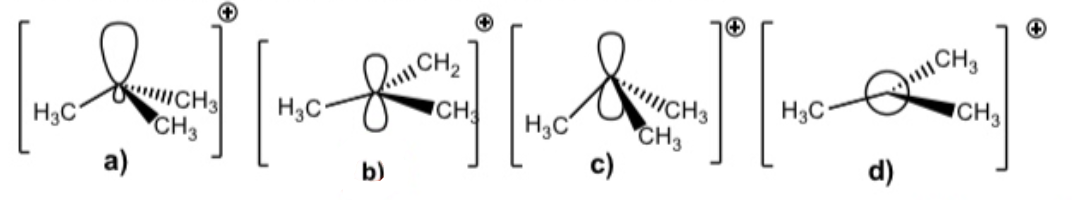
Select the accurate orbital representation of the tert-butyl cation
B
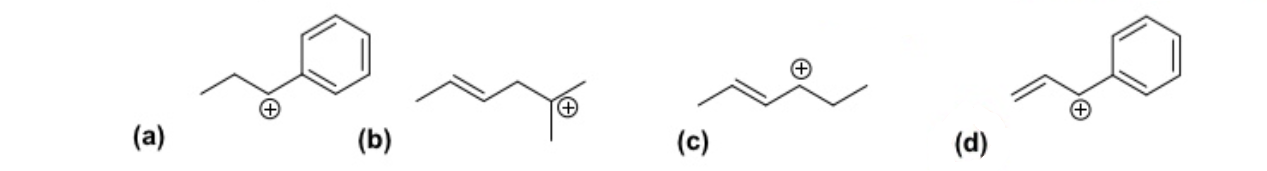
Which of the following carbocations is the most stable ?
D
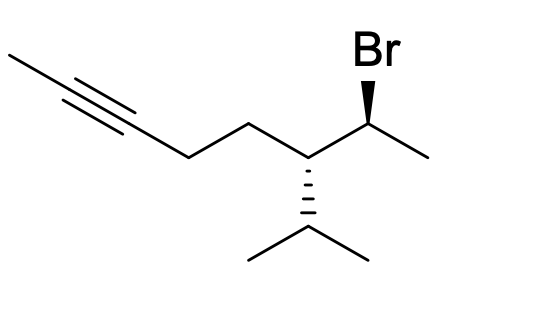
What is the IUPAC name of the following compound? (hint: check priority table)
(6S,7S)-7-bromo-6-isopropyloct-2-yne
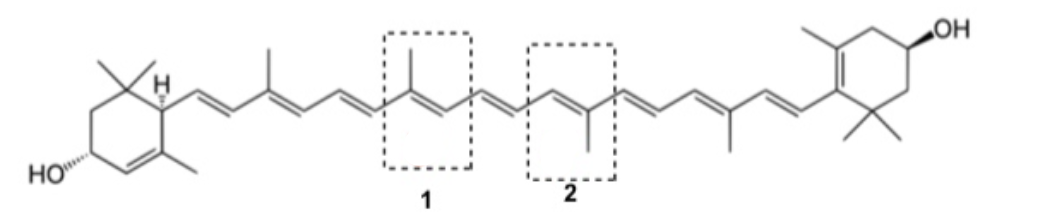
Lutein from Latin luteus meaning "yellow" is a xanthophyll and one of 600 known naturally occurring carotenoids. Assign E/Z stereochemistry of the C=C bonds circled and labelled as 1 and 2
1 = E; 2 = E
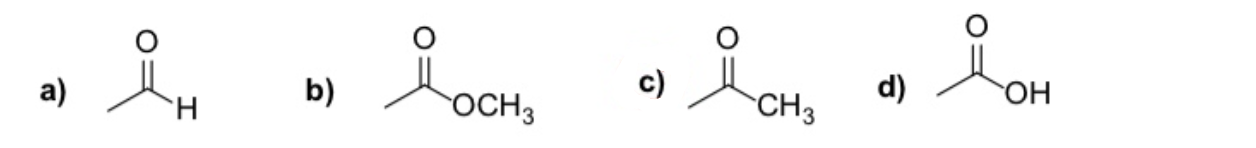
which of the following functional groups is a ketone
C
In class (especially in Ch. 6) we discussed examples of regioselectivity, regioselectivity, enantioselectivity and stereospecificity of reactions. Select the correct definition of stereoselectivity
Reactions that can give two different stereoisomers but one of those stereoisomers is the major or only product
What is the approximate pKa of a terminal acetylene R-C≡CH ?
25
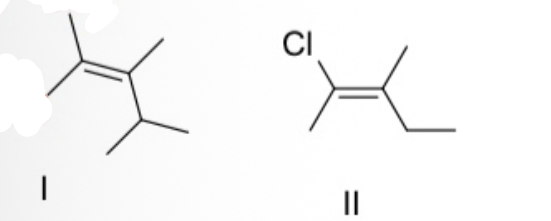
Pick the correct statement describing E / Z /neither configurations for the following two compounds.
Compound I = neither; Compound II = E.
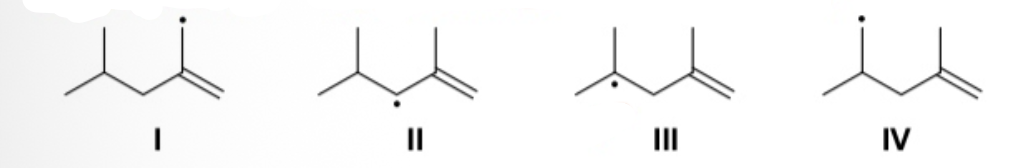
Rank the following radicals in order of decreasing stability, from most stable > to least stable.
II > I > III > IV
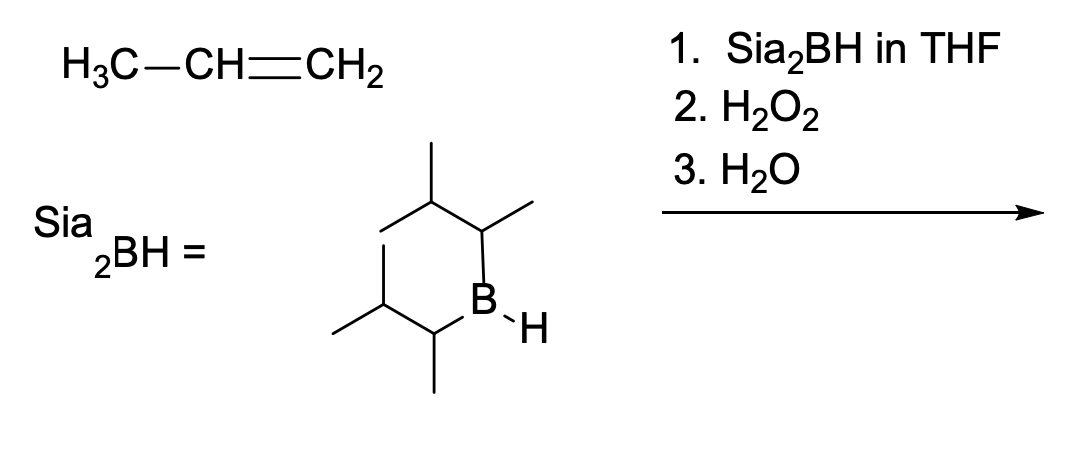
Which of the following species is formed at some point during this hydroboration/oxidation reaction?
B
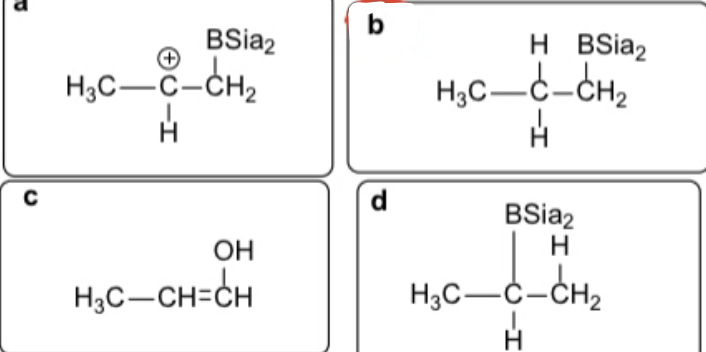
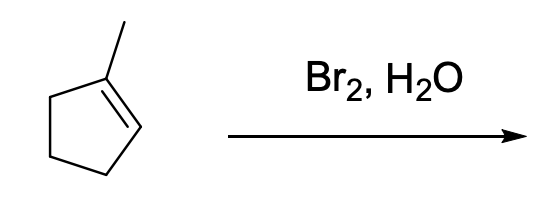
Select the correct products obtained in this reaction
D
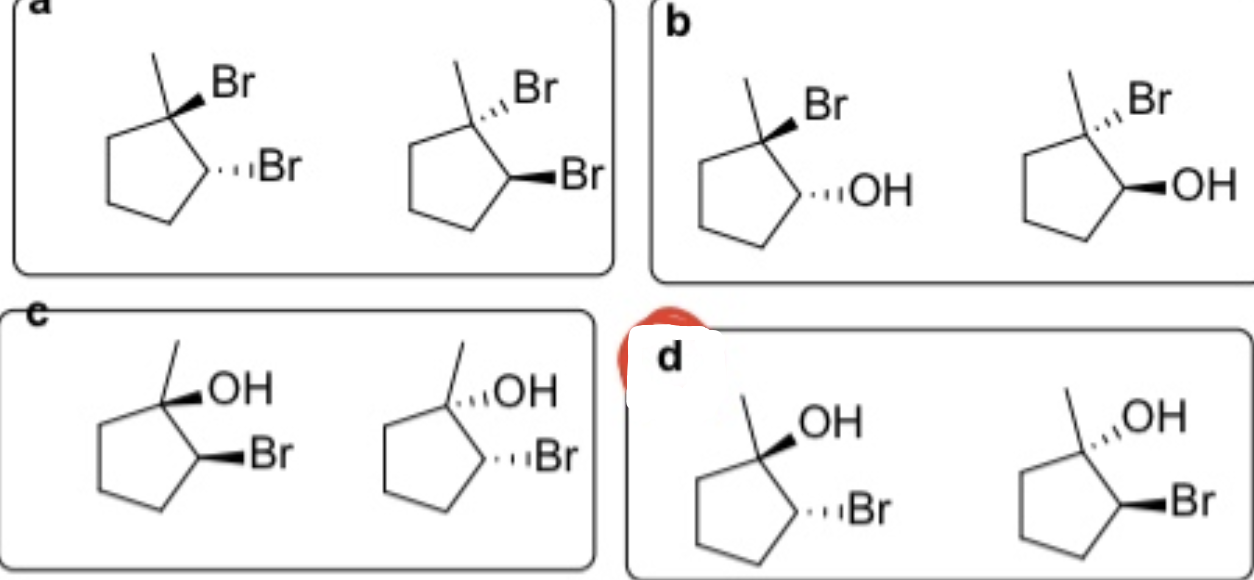
Which of the following reagents adds with ANTI stereochemistry?
Br2
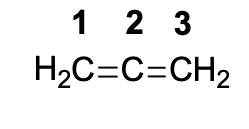
Select the correct description of this compound
It is an allene, it has a twisted structure, C2 is hybridized sp and C1 and C3 are hybridized sp2
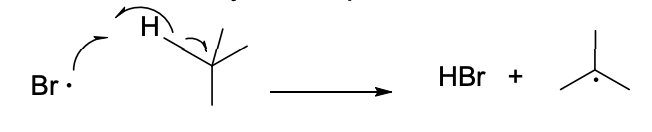
Identify this step.
It is the propagation step of a Radical reaction