AP Biology - Unit 1
Chemistry Foundations:
| Particle | Charge | Weight | Location |
|---|---|---|---|
| ==Proton== | +1 | 1 dalton | Nucleus |
| ^^Neutron^^ | 0 | 1 dalton | Nucleus |
| Electron | -1 | 0 daltons | Electron Shell |
Atomic Mass = Protons (= Electrons) + Neutrons
Most Important Biological Elements: %%Oxygen%%, %%Carbon%%, %%Hydrogen%%, %%Nitrogen%% (Calcium, %%Phosphorus%%, ==Potassium==, %%Sulfur%%, ^^Iron^^, %%Iodine%%, etc.)
Valence Electrons (Outer Shell) are the most important in determining properties
Covalent Bond - when electrons are shared between elements
- Polar - electrons aren’t shared equally
- Non-polar - electrons are shared equally
Ionic Bond - when electrons are transferred between elements
Bond Strengths - single (-), double (=), triple (≡), etc.
Isomers are molecules with the same formula but different shapes/properties:
- structural - different covalent arrangements
- cis-trans - different arrangement around a double bond
- enantiomers - mirrored
Water:
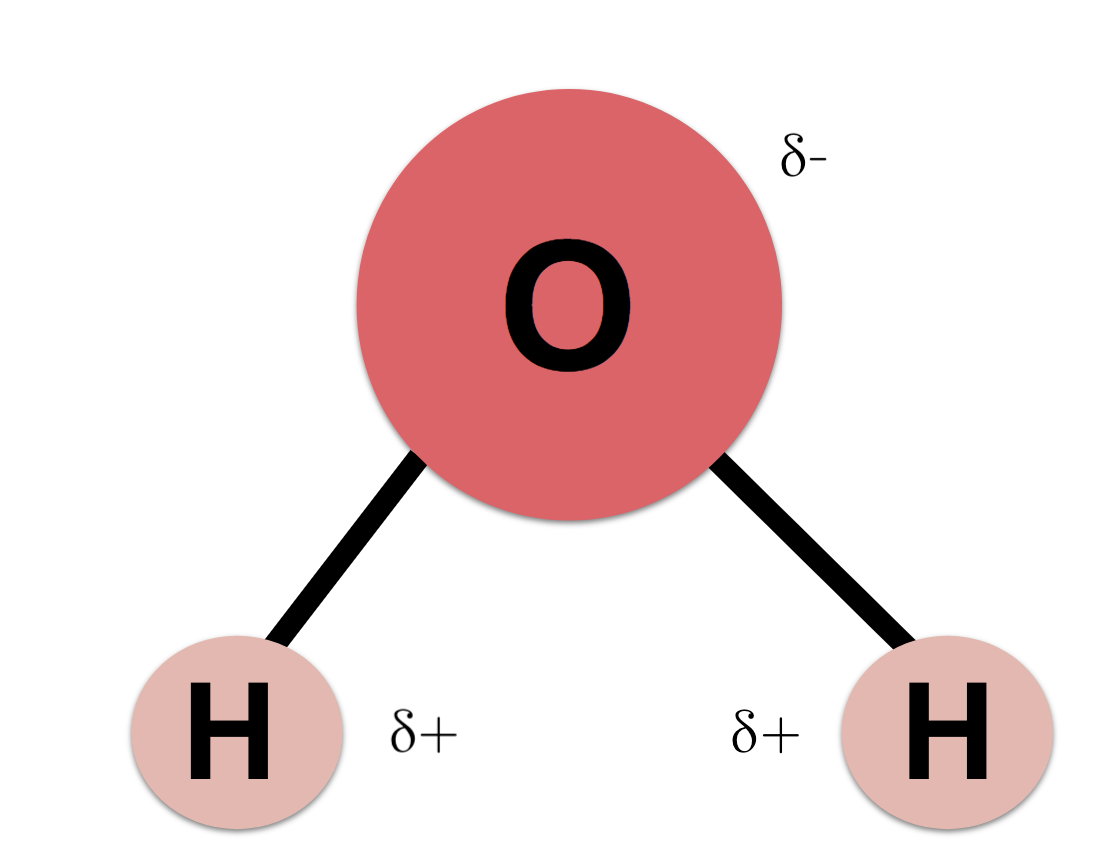 Cohesion - the molecules of water are attracted to one another because of the hydrogen bonds (due to polarity)
Cohesion - the molecules of water are attracted to one another because of the hydrogen bonds (due to polarity)
Adhesion - the molecules of water are attracted to other molecules because of the hydrogen bonds
Surface Tension - the cohesive properties of water are strong at the surface
High Specific Heat - since water is heat resistant, the amount of energy required to increase the heat is greater than most substances
High Heat of Vaporization - since water is heat resistant, the amount of energy required to vaporize it is high
Ice - when water freezes, the molecules spread further apart, which makes ice less dense than water
Compounds that are hydrophilic are attracted to water molecules, and compounds that are hydrophobic repel from water molecules
Acidity - sometimes, water molecules disconnect into H+ and OH-, more H+ makes water more acidic, more OH- makes water more basic, and if they are equal water is neutral
Biomolecules:
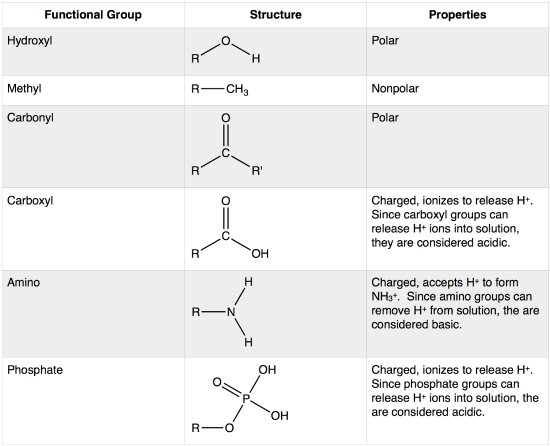
Functional Groups (common groups found in biomolecules) -
- Monomer: Monosaccharides
- Polymer: Polysaccharides
- Bond: Glycosidic (dehydration (condensation) reaction and hydrolysis)
- Structure: made of carbon chains and rings, contains carbon, oxygen, and hydrogen
- Properties: combustible
- Usage: energy use and storage (starch and glycogen)
Lipids -
- Monomer: Glycerol, Fatty Acids
- Polymer: Lipids
- Bond: Ester
- Structure: Fats (Glycerol + 3 Fatty Acids), Phospholipids (Glycerol + 2 Fatty Acids), Steroids (4 Carbon Rings), Wax (Fatty Acid Chain + Alcohol Chain)
- Properties: bilayers
- Use: energy storage
- Monomer: Nucleotides (Pentose Sugar, Phosphate Group (nucleoside), Nitrogenous Base)
- Polymer: Deoxyribonucleic Acid (DNA) or Ribonucleic Acid (RNA), also called Polynucleotides
- Bond: Phosphodiester (between sugar and phosphate group), Hydrogen (between nitrogenous bases)
- Structure: DNA - sugar (deoxyribose) attaches to phosphate group, and two chains connect in a double helix antiparallel shape with base pairs connecting (A-T, C-G); RNA - sugar (ribose) attaches to phosphate group to create a chain, and sometimes base pairs connect (A-U, C-G)
- Properties: N/A
- Use: stores and express genomic information to create proteins
Example: Adenosine triphosphate (ATP) stores energy and splits to release it
Proteins -
- Monomer: Amino Acids
- Polymer: Proteins, Polypeptides
- Bond: Peptide
- Structure: Primary (chain of amino acids), Secondary (α helix, β-pleated sheets; interactions between amino and carboxyl groups), Tertiary (Hydrogen Bonds, Ionic Bonds, Disulfide Bridges, Hydrophobic Interactions, etc.; interactions between R-groups), Quaternary (interactions between multiple polypeptides)
- Properties: changes between proteins
- Use: many different uses (enzymes speed up and are required for some biological chemical reactions)
- Other Notes: Proteins require very specific chemical conditions to form properly, and when this strays and the shape is altered, it is called denaturation
Review Resources: