DDS lecture 7 content
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
Emulsions
dispersion in which the dispersed phase is composed of small globules of a liquid, distributed throughout a vehicle in which it is immiscible
Oil in water (o/w) emulsion
oils, petroleum hydrocarbons, or waxes are dispersed in water
external phase: oil
internal phase: water
generally formed if the aqueous phase >45% of the total weight
hydrophilic emulsifier is used
emulsifier must be miscible with larger/external phase
Water in oil (w/o) emulsion
water or aqueous solutions are dispersed in an oleaginous medium
aqueous phase constitutes <45% of the total weight
lipophilic emulsifier is used
Stability
emulsions are thermodynamically unstable
separates into two phases
instability:
creaming (better than coalescence + breaking)
coalescence
phase inversion
must have appropriate emulsifier to prevent instability
Creaming
dispersed droplets merge and rise to the top or fall to the bottom of emulsion
result: lack of drug distribution
creaming in o/w emulsions characterized by oil globules gathering and rising to the top (oil is less dense than water)
reversible by shaking
is reversible due to protective film around droplets which prevent coalescence
Coalescence
breaking
irreversible - film around individual droplets are destroyed
viscosity alterations may help stabilize droplets and minimize tendency to coalescence
to avoid, use optimum phase: volume ratio
internal volume should be less than external volume
Phase inversion
change in type of emulsion (o/w into w/o, vice versa)
sign of instability
Emulsifying agents
emulsifying agents concentrate and are adsorbed at the oil:water interface to provide a protective barrier around the dispersed droplets
stabilize the emulsion by reducing the interfacial tension in the system
some also impart charge on droplet surface, reduce contact between droplets, decreasing potential for coalescence
Chemical structures classifications of emulsifying agents
synthetic
natural
finely dispersed solids
auxiliary agents
secondary emulsifying agent (support action of primary)
Mechanisms of action classifications of emulsifying agents
surface active agents (adsorb at o:w interface, form monomolecular layer)
hydrophilic colloids (forms multimolecular layers at interface)
finely divided solid particles (adsorp at interface and forms a layer of particles around the droplets)
Surfactants
synthetic emulsifying agents
hydrophilic and lipophilic part
moves to liquid: liquid interface, reducing surface/interfacial tension in the system
Anionic surfactants
synthetic emulsifying agent
soaps and detergents
subject to hydrolysis, less desirable than stable detergents
surface active groups contain carboxylate, sulfate and sulfonate groups
sodium lauryl sulfate (SLS)
long alkyl chain sulfonates less susceptible to hydrolysis
Cationic surfacetants
synthetic emulsifying agents
bactericidal
long chain amino and quaternary ammonium salts
both cationic and anionic emulsifiers used in topical o/w emulsions, but cationic less frequently used
Nonionic surfactants
synthetic emulsifyin agent
most frequently used
superior in compatibility, stability, lack of toxicity
neutral pH - resistant to addition of acid and electrolytes
most commonly used are polyoxyethylene sorbitan fatty acid esters
Span
Tween
Plant/Animal sources of emulsifiers
natural emulsifying agents
most from a hydrated lipophilic colloid (hydrocolloid)
form a multi-molecular layer around the emulsion droplets
little to no effect on interfacial tension
protective colloid effect and reduce potential for coalescence by
providing protective sheath around droplets
imparting charge to dispersed droplets
swelling to increase viscosity of system (less likely to merge)
Classified emulsifying agents
natural emulsifying agent
vegetable derivatives: acacia, tragacanth, agar, pectin, carrageenan
animal derivatives: gelatin, lanolin, cholesterol, lecithin
semi-synthetic agents: methylcellulose (MC), carboxymethylcellulose (CMC)
Synthetic: carbopols
Finely divided or dispersed solid particle emulsifiers
form particulate layer around the dispersed particle
most will swell and increase viscosity to reduce interaction between dispersed droplets
most o/w but some may work for w/o
ex. bentonite,- fatty acids (stearic acid) Veegum, magnesium hydroxide, aluminum hydroxide, magnesium trisilicate
Auxiliary emulsifying agents
Fatty acids (stearic acid)
Fatty alcohols (Stearyl or Cetyl alcohol)
Fatty esters (glyceryl monostearate)
Stabilizes emulsions by thickening formulation
weak emulsifying properties, thus used in combination with other emulsifiers
Hydrophile-Lipophile balance (HLB)) system
system developed to assist in deciding how much and types of surfactants to add to make an emulsion
arbitrary scale
numbers determined experimentally
low number = few hydrophilic groups, more lipophilic
Span: oil soluble, used for w/o emulsion
High number = large number of hydrophilic groups, more hydrophilic character
Tweens: water soluble, used for o/w emulsions
Span
emulsifying agent for water in oil emulsions
oil soluble
low HLB
non-ionic surfactant
Tween
emulsifying agent for oil in water emulsions
high HLB
water soluble
non-ionic surfactant
HLB value for emulsifying agents for w/o emulsions
3-8
HLB value for emulsifying agents for o/w emulsions
8-16
HLB value for detergents
13-16
HLB value of surfactant system
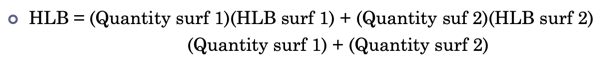
Continental/Dry gum/4:3:2 method of compounding emulsions
prepare initial emulsion from oil, water and vegetable derived hydrocolloid or gum type emulsifier (usually acacia)
primary emulsion = 4 parts oil, 2 parts oil, 1 part emulsifier
Emulsifier triturated with oil until powder wetted thoroughly
add water, vigorously triturate
creamy white emulsion made
English/wet gum method
emulsifier triturated with water
oil added in portions while triturating
more difficult than dry gum, but yields more stable emulsion
4 parts oil, 2 parts water, 1 part emulsifier
Adding ingredients to primary emulsion
solid substance
API preservative, colors, generally dissolve and added as solution to primary emulsion
volatile ingredients added when product is cool
substances which may reduce physical stability
ex. alcohol, which will precipitate the gum, should be added near end
after incorporation of all ingredients, blend to ensure uniform distribution of medicaments
viscosity enhancers can be added to increase stability
o/w: hydrocolloids
w/o: viscous oils, fatty alcohols, fatty acids
Adding ingredients to commercial products (w/o)
w/o emulsions, oils, and insoluble powders can be incorporated directly into external phase with spatula and pill tile or low heat
adding aqueous soluble materials to w/o emulsion more difficult
excess emulsifier must be present to accommodate more water
aqueous solution of drug added to emulsion with pill tile and spatula
Adding ingredients to commercial product (o/w)
o/w emulsions - good levigation agents for aqueous insoluble substances needed
ex. glycerin, propylene glycol, polyethylene glycol (PEG) 300 or 400, alcohol
use salt form of API if available
external phase incorporation is easy with pill tile and spatula
heat may be needed
Flavoring emulsions
for o/w emulsions, if using flavoring oil, it may concentrate into internal phase
strength in external phase is less
to reduce partitioning, flavoring oils may be mixed with small amount of emulsifier, then added
3-5 times as much emulsifier as oil should be used
or mix with ethanol or glycerin
Determination of emulsion type
dilution test
dye test
drop test
Dilution test
dilute with water
o/w emulsion will be stable
w/o will separate and break
Dye test
add water soluble dye to sample and mix
if uniform color, o/w emulsion
globular distribution shows w/o emulsion
Drop test
put drop of emulsion on water
if spread out - o/w bc miscible
if stays drop - w/o
Antimicrobial additives
methylparaben
propylparaben
benzoic acid
benzalkonium chloride
Antioxidant additives
Ascorbic acid
butalyted hydroxyanisole (BHA)
butylated hydroytoulene (BHT)
1-tocopherol
liquid in liquid
emulsion
solid in liquid
suspension
solid in solid
mixture (powders and granules)
Suspensions
a heterogenous mixture in which the internal phase is dispersed throughout the external phase through mechanical agitation, with. the use of certain excipients or suspending agents
two-phase system consisting of undissolved or immiscible material dispersed in a vehicle
will eventually settle
Advantages of suspensions
chemical stability
some compounds stable when suspended but unstable when dissolved
organoleptic reasons
disagreeable tastes/smells often attenuated when in suspension vs solution
ease of swallowing
liquid
easier for infants, children, elderly patients
higher bioavailability
Bioavailability order
Solution > Suspension > Capsule > Compressed Tablet > Coated Tablet
Disadvantages of suspensions
physical stability, sedimentation, and compaction may cause problems
bulky
sufficient care must be taken during handling, transport
difficult to formulate
uniform and accurate dose cannot be achieved unless suspension packed in unit dosage form
Ideal properties of suspensions
settle slowly and readily disperse upon gentle shaking
should not be stirred
particle size remains constant throughout long periods of standing
pour readily and evenly from its container
physically and chemically stable
resistant against microbial contamination
good organoleptic properties
Applications of suspensions
applicable for insoluble/poorly soluble drug
prevent degradation of drug or improve stability of drug
mask unpleasant/bitter taste of drug
can be formulated for topical application
can be formulated for parenteral application
control rate of drug absorption
vaccines, X-ray contrast agents
Routes of administration of suspensions
oral suspensions
topical suspensions
parenteral suspensions
ophthalmic suspension
Stokes’ law
speed with which particles settle out of a liquid medium is dependent on a constant factor (K) and the radius of the particles
the bigger the particle, the faster it will fall out of suspension
particle moving through viscous liquid attains constant velocity (sedimentation rate)
very slow for particles with density close to liquid density, particles with small diameter, or where viscosity is high
Stokes’ Equation
dx/dt = rate of settling
d = diameter of particle
Pi = density of particles
Pe = density of medium
g = gravitational constant
N = viscosity of medium
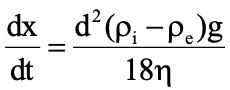
Factors affecting sedimentation
particle size diameter
smaller diameter = slower rate
density difference between dispersed phase and dispersion media
lower density difference = slower sedimentation rate
viscosity of dispersion medium
increase in viscosity = decrease settling
greater increase in viscosity leads to issues like pouring, syringibility, redispersibility of sediments