AQA A LEVEL Physics Discovery of the Electron
1/12
Earn XP
Description and Tags
🔬 Background Context In the late 19th century, atoms were thought to be indivisible. Experiments with cathode rays (streams of particles emitted from the negative electrode in vacuum tubes) led to new discoveries. ⚡ Key Experiments and Discoveries 1. Cathode Ray Tubes Observed glowing rays from cathode to anode in evacuated tubes. Rays cast shadows and could turn a paddle wheel – suggesting they had mass and traveled in straight lines. Were deflected by electric and magnetic fields → showed they were negatively charged particles. 2. J.J. Thomson’s Experiment (1897) Used electric and magnetic fields to deflect cathode rays in opposite directions. Balanced the electric and magnetic forces to find the velocity and then the specific charge (e/m). 🔍 Key Result: Found e/m = 1.76 × 10¹¹ C/kg, much larger than for ions. Showed cathode rays were not atoms but tiny negatively charged particles → electrons. 3. Millikan’s Oil Drop Experiment (1909) Measured the charge of the electron. Observed tiny charged oil droplets suspended in an electric field. Balanced gravitational force and electric force to find e ≈ 1.6 × 10⁻¹⁹ C. 🔍 Combined with Thomson’s e/m: Calculated the mass of the electron ≈ 9.11 × 10⁻³¹ kg. 🧠 Conclusions and Impact Electrons are fundamental subatomic particles. Discovery overturned the idea of the atom being indivisible. Led to the plum pudding model, and eventually Rutherford’s nuclear model.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
How are cathode rays made in a discharge tube?
Electrons are released by thermionic emission.
Electrons are repelled by cathode and accelerated towards anode.
Why is light emitted from a discharge tube?
A strong p.d. between the anode and cathode cause atoms in the discharge tube to ionise.
When these positive ions are attracted to the cathode, they accelerate and collide with the cathode.
This causes electrons to leave the cathode and go to excite other atoms, when those atoms excite and de excite they release photons of light.
The speed, v, of each electron leaving the anode in a cathode ray?
Work done on each electron is equal to the kinetic energy gained, which is given by the equation W = qV, where q is the charge of the electron and V is the potential difference. (gain in Ek=Loss of Ep)
This increases the speed of each electron as it accelerates to the anode.
State 3 methods used to work out the specific charge of an electron
Using a magnetic field.
r=mv/Be
e/m=v/Br
e/m=2V/B²r²
Why must gas pumped into a electron tube be low?
Low pressure = less molecules.
To ensure that collisions between gas molecules and electrons are minimized, allowing for efficient electron acceleration and less scattering.
This could result in electrons not being able to travel the entire length of the tube.
Who was Thomson and what did he accomplish?
Conducted experiments investigating cathode rays which lead to him discovering the electron and it’s specific charge.
What did Thomson determine about the specific charge of an electron in comparison to an hydrogen ion? Why was it significant?
Thomson determined the specific charge of an electron was 1800x greater than a hydrogen ion.
This meant the electron had either a massive negative charge or a very small mass.
Significance: hydrogen was previously determined to have the highest specific charge of any particle, indicating that the electron is a fundamental component of matter.
Who was Millikan and what was the significance of his experiment?
Robert Millikan was an American experimental physicist renowned for his oil drop experiment, which measured the charge of the electron.
He found that the common factor between the charges found on the droplets was no less than the elementary charge(1.6×10^-19) so he determined that the charge of all material is quantised.
What forces act on a droplet in Millikan’s experiment when stationary?
mg=eV/d
The weight of the droplet acting down, and the Electric force which is equal to the weight, acting upwards to balance it.
Explain the journey of a falling droplet including how to cause the droplet to fall from stationary.
A falling droplet begins stationary due to the balance of forces acting on it.
When the p.d. is decreased, the electric force acting upwards becomes weaker, allowing the weight of the droplet to cause it to accelerate downwards.
If the p.d. is shut off completely, the weight of the droplet causes it to accelerate downwards (\/)
As it falls drag increases reducing the rate of acceleration until mg=drag then it has reached it’s terminal velocity.
What did Stoke accomplish?
Stokes formulated the law of viscosity, which describes the drag force experienced by a sphere moving through a viscous fluid, highlighting the relationship between the droplet's size, velocity, and the fluid's viscosity.
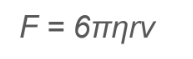
How does Stoke’s Law determine the radius of a droplet?
mg= 6πrηv, where r is the radius of the droplet, η is the fluid's viscosity, and v is the velocity.

How did Millikan use the value of the radius to determine the charge of an electron?
He used the radius to determine the mass of the droplet, to calculate it’s weight.
He then was able to determine the p.d. necessary for the droplet to remain stationary.
From that he could calculate the charge using eV/d=mg.