Electrolytic cells personal notes
0.0(0)
0.0(0)
Card Sorting
1/9
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
10 Terms
1
New cards
Electrolysis
A process by which an electric current breaks chemical bonds. Forcing a non spontaneous redox reaction to occur
2
New cards
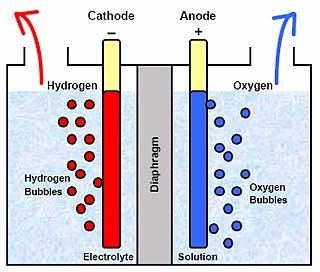
Electrolysis of water
The decomposition of water into oxygen and hydrogen gas due to an electric current being passed through the water
3
New cards
The external power supply
Acts as an electron pump, the electric energy is used to do work on the electrons to force an electron transfer inside an electrolytic cell
4
New cards
Products + electrical energy
reactants
5
New cards
An electrolytic cell has a ___________ cell potential
negative
6
New cards
What is required to force the reaction?
an applied voltage of at least the absolute value of the standard cell potential. Nothing less or the reaction will not occur.
7
New cards
secondary cell
A cell that can be recharged
(ex: nickel cadmium cell).
(ex: nickel cadmium cell).
8
New cards
as the secondary cell discharges
electrical energy is spontaneously produced and the cell functions as an electric cell
9
New cards
when the secondary cell is recharged
the electrical energy forces the products to react and re form the original reactants. During this time the cell is functioning as an electrolytic cell.
10
New cards
molten salt electrolysis
metal cations move to the cathode and are reduced to metals and non metal anions move to the anode and are oxidized to non metals.