Treatment and Room Design, Patient Safety, Data Transfer, Professionalism
1/419
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
420 Terms
Emami Paper
presented a dose for different treatment sites at which 5% of patients would suffer a complication.
did not consider concurrent chemo
did no use rx or calcs with heterogeneity calcs
mostly 3D
QUANTEC Constraint and Endpoint: Brain
Endpoint: Necrosis (death of tissue)
3D-CRT: Dmax < 60 Gy (<3% complication rate)
SRS (Single Fraction): V12 (volume receiving 12 Gy) < 10 cc (<20% complication rate)
QUANTEC Constraint and Endpoint: Brainstem
Endpoint: Necrosis or neuropathy (damage to a nerve resulting in loss of associated function)
3D-CRT: Dmax < 54 Gy (<5% complication rate)
SRS (Single Fraction): Dmax < 12.5 Gy (<5% complication rate)
QUANTEC Constraint and Endpoint: Optics/BrainChiasm
Endpoint: neuropathy (optic to be precise)
3D-CRT: Dmax < 55 Gy (<3% complication rate)
SRS (Single Fraction): Dmax < 12 Gy (<10% complication rate)
QUANTEC Constraint and Endpoint: Spinal Cord
Endpoint: myelopathy (spinal cord dysfunction)
3D-CRT: Dmax = 50 Gy (0.2% complication rate)
SRS (Single Fraction): 13 Gy (1% complication rate)
SRS (Hypofractionation): 20 Gy (1% complication rate)
QUANTEC Constraint and Endpoint: Chochlea
Endpoint: sensory hearing loss
3D-CRT: Mean Dose < 45 Gy (<30% complication rate)
SRS (Single Fraction): prescription dose < 14 Gy (<25% complication rate)
QUANTEC Constraint and Endpoint: Parotids
Endpoint: loss of parotid salivary function
3D-CRT: Mean Dose < 25 Gy (<20% complication rate)
QUANTEC Constraint and Endpoint: Lungs
Endpoint: pneumonitis
3D-CRT:V20 < 30% (<20% complication rate)
QUANTEC Constraint and Endpoint: Esophagus
Endpoint: esophagitis
3D-CRT: Mean Dose < 34 Gy (5-20% complication rate)
QUANTEC Constraint and Endpoint: Heart
Endpoint: pericarditis (inflammation of the pericardium)
3D-CRT: V25 < 10% (<1% complication rate)
QUANTEC Constraint and Endpoint: Liver
Endpoint: Hepatitis and RILD (Radiation Induced Lung Disease - can involve pneumonitis and fibrosis. Note: this is for the right lung for a whole liver GTV)
3D-CRT: Mean Dose < 30 Gy (<5% complication rate)
SRS (Hypofractionation): Mean Dose < 13 Gy (<5% complication rate)
QUANTEC Constraint and Endpoint: Kidneys
Endpoint: renal dysfunction
3D-CRT: V20 < 32% (<5% complication rate)
QUANTEC Constraint and Endpoint: Small Bowel
Endpoint: bowel toxicity diarrhea
3D-CRT: V15 < 120 cc (<10% complication rate)
QUANTEC Constraint and Endpoint: rectum
Endpoint: rectal toxicity (fistulas)
3D-CRT: V50 < 50% (<15% complication rate)
QUANTEC Constraint and Endpoint: bladder
Endpoint: toxicity (loss of urinary control)
3D-CRT: V65 < 50%
QUANTEC Constraint and Endpoint: penile bulb
Endpoint: erectile dysfunction
3D-CRT: V50 < 90% (<35% complication rate)
ACR-ASTRO definition of SRS
radiation therapy delivered via stereotactic guidance with approximately 1 mm targeting accuracy to intracranial targets in 1-5 fractions.”
ACR-ASTRO definition of SBRT
radiation therapy delivered via stereotactic guidance with high levels of targeting accuracy to extracranial targets.” Furthermore, “SBRT is typically a complete course of therapy delivered in 1 to 5 [fractions].”
Dose Delivery Accuracy Requirement: for SRT
3%
size of tumor common requirements for SRS/SBRT
normally confined to being less than 4-5 cm in diameter
QMP responsibilities for SBRT as outlined in MPPG9
QMP must be available for consult from start (simulation) to finish (treatment delivery) (taken from ACR-ASTRO).
QMP must provide or oversee acceptance testing and commissioning including:
TPS validation,
Small field dosimetry,
Heterogeneity calculations (if applicable),
IGRT/localization evaluation, and
E2E testing.
Develop an ongoing QA program.
Develop and collaborate for the composition of standard operating procedures (SOPs).
Develop safety checklists.
Incorporate an incident learning system (for example, the Radiation Oncology Incident Learning System, RO-ILS).
Supervise or perform treatment planning (if performing, adequate secondary plan evaluation must be in place).
Initial and final plan review (if performing the planning, adequate secondary/independent plan evaluation must be in place).
Provide appropriate plan-specific QA (including dosimetric and “dry run” verifications).
TG-100 and MPPG 9 both explicitly recommend that the QMP provide personal supervision (defined above) for the entirety of the first fraction. Following that, MPPG 9 states that subsequent fractions can minimally be under direct supervision (defined above) by the QMP.
CT sim slice thickness for SRS and SBRT
For SBRT: slice thickness of 1-3 mm.
For SRS: no greater than 1.25 mm.
PDDs and beam profile measurements for SRT QA
you should use your smallest chamber available (needs to have a diameter < 1mm.
ICRU conformity index and common value for SRS
ratio of the volume enclosed by the prescription isodose surface and the planning target volume.
between 1 and 1.6 for SRS
Paddick conformity index
does not distinguish between over-coverage and under-coverage. The value will always be less than or equal to 1.0 but greater than or equal to 0.0 by mechanism of the formula
TVPIV is target volume covered by rx isodose volume
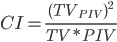
CI50
used to assess intermediate dose falloff
This value is the ratio of the volume receiving 50% of the prescription dose to the volume of the PTV.
A general target goal for CI50 for lung SBRT plans should be less than 5-6.
Gradient Index
unitless value
ratio of volumes of half he rx isodose to entire rx dose
should be less than 5 (ideally 3-4)
Homogeneity Index
the inverse of the prescription isodose line and is a general indicator of the uniformity (or, conversely, non-uniformity) of the dose across the target volume. In equation form, it is equal to the maximum dose divided by the prescription dose.
should be greater than 1 and can approach 2
Heterogeneity Index
Ratio of highest dose received by 5% of PTV to the lowest dose received by 95% of PTV.
Other SBRT common important metrics
V12 in the brain
V20 in lung
D2cm lung
what guidelines/TG reports handle SRS/SBRT
MPPG 9: SRS-SBRT
TG-135: Quality assurance for robotic radiosurgery
TG-42: Stereotactic radiosurgery
TG-101: Stereotactic body radiation therapy
CSI LINAC 3D setup
LINAC-based (patient is setup prone).
A pair of lateral fields are used to treat the brain and upper cervical spine.
For adults, 2 PA fields are used to treat the spinal column due to the length.
This necessitates a junction on the spinal cord which is usually matched at the anterior of the cord (for a cold match).
For pediatric cases, the entire spinal column can usually fit within a single field.
Both the cranial and spinal junctions are moved every few fractions to ensure that any hot and cold spots are distributed and not concentrated in one area.
Number of junctions needed in CSI tx
Junction # = tumor dose/(tolerance-dose)
Field matching with CSI: what needs to be matched and by how much
The cranial field has to be matched in 2 places: at upper spine and at cranial themselves
to do so rotate the collimator for spine field
can do half beam block or kick couch for cranials
when using 2 spine fields in 3D CSI how do we match them
cold match safest - vary the junction
need to know the depth of cord
TSE: areas that commonly need to be boosted
The soles of the feet
The palms of the hand
The top of the scalp
The perineum and anal area
TSE: commonly shielded areas
top of scalp
eyes
nails
How are upper and lower fields used for TSE
upper and lower fields are used separated by about 20 degrees. The air and plexiglass degrader function to scatter the electrons into a uniform field within (+/- 8% vertical and +/- 4% horizontal).
There is another added benefit to the angled fields in that most of the x-ray contamination is concentrated around the central axis. Having two angled fields then directs this above and below the patient (x-ray contamination above 4% is considered unacceptable
what is the function of the plexiglass spoiler for TSE
degrades and lowers the energy so electrons do not penetrate as far
scatters electrons to produce a more uniform field
Ideally what should the dose uniformity be for a TBI
Dose uniformity should be within +/-10% over the whole body.
What nodes are commonly looked at for involvement in breast cancer and potentially treated
Level 1 nodes (Axillary Nodes)
Level 2 nodes (Axillary Nodes)
Level 3 nodes
Supraclavicular nodes
Internal mammary nodes
Breast treatment fields setup for a 2 field plan
6x is usually used to keep the skin dose high, but for large patients, a mix of 6x and 18x may be used to reduce hot spots.
The posterior border of the fields is usually matched to remove divergence reducing dose to the lung and heart.
The collimator is turned (about 8 degrees) to match the angle of the chest wall and fields extend about 1-2 cm into the lung.
The fields should cover all the palpable breast tissue plus a 2 cm margin on all sides.
Care should be taken to not cross the midline and expose contralateral breast tissue to unnecessary radiation (induces secondary cancers).
Wedges or field-in-field planning may be performed to reduce hot spots.
Note that a conventional hard wedge produces significant scatter and will increase dose to the contralateral breast by about 2.5%.
FiF planning is essentially a step and shoot IMRT plan that uses a forward optimization process. That is the planner designs the MLC movements.
Breast 3 field plan field design
The patient's head is normally turned away (to keep the chin out of the field) from the field which is then angled 10 degrees to reduce cervical spine dose and the cord is blocked using MLCs.
The most important facet of these plans is matching the tangents and the supraclav field (SCV field).
If the plan uses separate isocenters for the tangents and SCV fields, then the couch can be kicked for the tangents to have matched upper borders. MLCs are then used to collimate the tangents to match the SCV field.
Another option is to use the same isocenter for the SCV and tangent fields which would then be half beam blocked by definition removing the divergence and thereby simplifying the matching.
breast 4 field plan
3 field with higher dose to the sclav field (4th post field)
breast 5 field plan
boost to the IMNs
Different breast “balloon” options
mammosite: balloon with central channel
filled with saline and contrast
have a new multilumen with 4 channels
Contura
5 channels
vacuum to help with removing air and seroma
SAVI
no balloon just struts
how does contrast in the breast balloon affect the dose
lower the dose compared to TG-43 by up to 5% (but limiting the Iodine contrast agent concentration to 10% reduces the dose discrepancy to below 3%)
Breast balloon dose constraints
Skin must be kept below 425 cGy (the minimum acceptable skin distance is usually about 5-7 mm).
Rib must be kept below 425 cGy (or risk fractures).
Draw and label the different treatment fields for hodgkins
Heterotopic ossification RT
delivery must come within hours of the operation associated with the trauma surgery.
It is for this reason that these patients must be treated emergently.
700-800 Gy
most common in hips
I-131 ablation therapy
The thyroid is the only organ in the body that requires iodine, and therefore any administered I-131 accumulates in the thyroid in great quantities.
The amount of I-131 that is delivered varies and depends on the following:
30-150 mCi is administered for primary tumors following resection.
150-250 mCi is administered for metastases or recurrences.
more precise way is to look at uptake with I-124 PET
I-131 isotope properties
beta emitter (606 and 334 keV) decaying to a metastable state of xenon which emits high energy gammas (364 and 636 keV).
I-131 release criteria
Release of the patient based on activity. For I-131, you can release the patient if the activity is no greater than 33 mCi. Or,
Release of the patient based on measured dose rate. You can release the patient, even if they received an activity greater than 33 mCi, provided that the dose rate at one meter from the patient is no greater than 0.07 mSv/hr (specific to I-131). Or,
Release of the patient based on patient-specific dose calculations. For these criteria, one has to perform patient-specific dose calculations to demonstrate that no dose to an individual is likely to be greater than 5 mSv. Furthermore, clear instructions should be provided to the patient to help them keep to that goal (for example, with instructions for how long to sleep in a separate bed from a spouse). The details of this calculation are provided in the cited NUREG regulatory document.
If the I-131 patient cannot be released what considerations need to be made for their hospital stay
Dose to patients in adjacent rooms is of concern and shielding the treatment room will likely be required.
The entire room must be covered in plastic sheeting that will be disposed of after patient discharge, as I-131 will be coming out of every pore on the patient.
Any nursing staff must wear dosimeters along with any visitors.
All staff involved with care and cleanup for the treatment must undergo a bioassay to assess the amount of radioactive iodine their thyroids took up.
Once dose rate readings fall below 0.07 mSv/hr at 1 meter the patient may be released with instructions to minimize dose to others (NUREG 1556).
The overarching goal when releasing any brachytherapy patient is to keep the dose to the maximally exposed individual below 5 mSv.
Hippocampal sparing WBRT dose constraints
Greater than 98% of the PTV (brain) receiving 25 Gy
Less than 2% of the PTV (brain) receiving 37.5 Gy.
Hippocampus max dose of 16 Gy.
100% of the Hippocampus receiving less than 9 Gy.
Optic Nerves and chiasm receiving less than 37.5 Gy.
storing radioactive material from patient waste and cite source
10 half lives
10CFR35
Photon beams spectrum:
max E
average E
max: designated energy
avg: 1/3
PDD for 10×10 10cm depth
Co
6
10
18
Co-60 = 55%
6X = 65%
10X = 75%
18X = 80%
TMR 10cm depth
6
10
18
6X = 0.78
10X = 0.85
18X = 0.90
attenuation per cm 6/10/18
6X = 3% per cm
10X = 2.5% per cm
18X = 2% per cm
dmax for 10×10
Co
6
10
18
Co-60 = 0.5 cm
6X = 1.5 cm
10X = 2.4 cm
18X = 3.3 cm
surface doses
Co
6
10
18
Co-60 = 50%
6X = 25%
10X = 23%
18X = 20%
neutron contamination 18 x beam
0.5% on the central axis
0.15% outside of the beam
neutron head leakage 6/10/18
6X = none
10X = 0.01%
18X = 0.15%
Scatter at 1 meter from a phantom
1/1000 primary beam
Lateral Scatter has a maximum energy
511 keV
backscattered radiation
255 keV
Photon interaction proportionalities per unit mass
Compton scattering - Z independent, proportional to electron-density.
Photoelectric interactions - proportional Z3.
Pair production - proportional to Z.
Conversion factor for Roentgens to cGy (f-factor)
air (MV)
tissue (MV)
tissue I-125
air: 0.876 cGy/R.
tissue: 0.97
tissue I125: 0.886
SRS treat to for linac and gamma knife
LINAC based therapy is prescribed to about the 80% isodose line.
Gamma Knife therapy is prescribed to the 50% isodose line.
scatter contributions of physical and dynamic wedges
Physical wedges - scatter about 2.5% the central axis dose.
Dynamic wedges - scatter about 1.0% the central axis dose.
extending perpendicular beam at dmax, magnitude at 10/30 cm
10 cm away receives approximately 1% of the central axis dose at dmax.
30 cm away receives approximately 0.2% of the central axis dose at dmax.
electron depth max dose

electron 80% depth
E/3
electron range in water
Rp = E/2
depth of 50% isodose line
E/2.4
depth max dose for 10×10 FS common electron E
6E = 1.2 cm
9E = 1.9 cm
12E = 2.1 cm
20E = 2.5 cm
electron surface dose: 6/12/20
6E = 80%
12E = 90%
20E = 95%
electron xray contamination: 6/12/20
6E = 1%
12E = 2%
20E = 5%
Amount of lead shielding needed in millimeters to stop an electron beam
E0(MeV) / 2.
occupation and public limits and avg worker exposure
Occupational limit = 50 mSv/year (5 mSv if pregnant).
Public limit = 1 mSv/year.
The average worker in the USA receives 2 mSv/yr from occupational exposures.
Neutrons produced in photonuclear reactions energy
about 2 MeV
concrete TVL for 6/18x
6X - 37 cm
18X - 45 cm
amount of lead in centimeters necessary to shield an electron beam (to less than 5%)
divide the practical range by 10.
avg exposure per year for average person
Radon - 1.0 mSv/yr
Ingested isotopes - 0.4 mSv/yr
Isotopes occurring naturally - 0.4 mSv/yr
Cosmic rays - 0.4 mSv/yr
Medical exposures - 2.0 mSv/yr
adding up to about 4-5 mSv per year for the average person:
k edge of lead and iodine
lead is 88 keV and Iodine’s is 33 keV.
Increase in mortality risk due to an acute and chronic radiation exposure
acute: 8% per Sv
chronic: 4% per Sv
radiation syndrome dose levels
Hematopoietic syndrome - 2 Sv
GI syndrome - 10 Sv
CNS syndrome - 30 Sv
Radiation skin effects dose levels (acute exposures):
Erythema - 6 Sv
Wet Desquamation - 25 Sv
Radionecrosis - 50 Sv
Radiation weighting factors:
Photons = 1
Electrons = 1
Protons = 5 (some discrepancy here with a range of 2-10 quoted with the NRC quoting 10 via 10CFR20)
Neutrons = 5-20 (10 for unknown energy)
Alpha = 20
Package Shipping Labels
White 1: <0.5 mrem/hr at surface
Yellow 2: <50 mrem/hr at surface, <1 mrem/hr at 1 m
Yellow 3: <200 mrem/hr at surface, <10 mrem/hr at 1 m
Package receiving wipe test action limits (for a 300 cm2 area):
Alpha - 22 dpm/cm2
Beta/gamma - 220 dpm/cm2
ALARA levels
ALARA level 1: 10% the allowed limit per quarter
ALARA level 2: 30% the allowed limit per quarter
sealed source leak test limits
more than 185 Bq of leakage then it must be removed from service (remember sealed sources must be checked at least every 6 months)
Patient exposure release levels
I-125
Pd-103
I-131
I-125: <1 mR/hr at 1 meter
Pd-103: <3 mR/hr at 1 meter
I-131: <7 mR/hr at 1 meter
brachy medical events
Wrong patient, site or nuclide
Total dose differs by 20%
Single fraction differs by 50%
teletherapy medical events
Wrong patient or site
Total dose differs by 20% (for treatments >3fx)
Total dose differs by 10% (for treatments
< 3fx)
Co-60 Energy, gamma, half life, HVL lead
1250 keV
13.07
5.3 years
1 cm
Ra-226 Energy, gamma, half life, HVL lead
830
8.25
1600 years
0.7 cm
Cs-137 Energy, gamma, half life, HVL lead
660 keV
3.26
30.2 years
0.55 cm