Ionic and covalent bonding- Yr 9
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
What is an anion?
A negatively charged ion
What is a cation?
A positively charged ion
How do you know if an ion isn’t stable?
The outer shell isn’t complete
How do you bond two ions together?

Why do ions have brackets around them with a -1?
It shows they have gained an electron, so that electron has given it a negative charge. Hence the -1
Why do ions have brackets around them with a +3?
The + shows it has lost something negative, so it regains a positive charge. Hence the +3
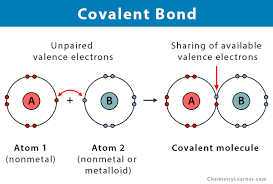
What is covalent bonding?
When atoms share pairs of electrons
What are 2 things that must happen in a covalent bond?
Must be between non-metal atoms
Always two atoms in one shared bond
What is an ionic bond?
A bond between metals and non- metals
How do you draw a covalent bond?