Topic 6. Chemistry A level Edexcel (copy)
1/84
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
85 Terms
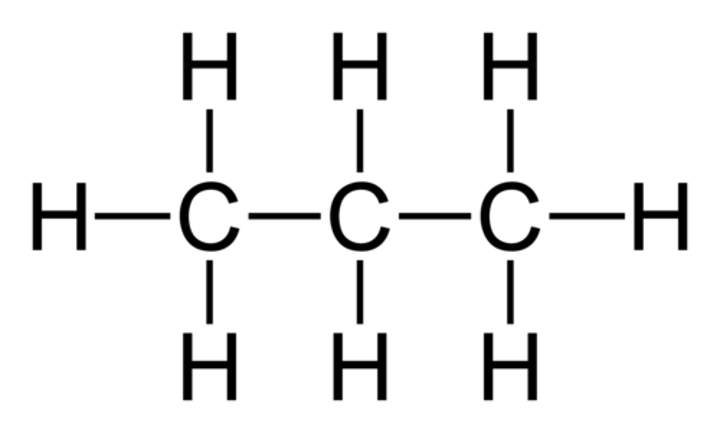
Displayed Formula
Shows all of the atoms in the molecule and their arrangements
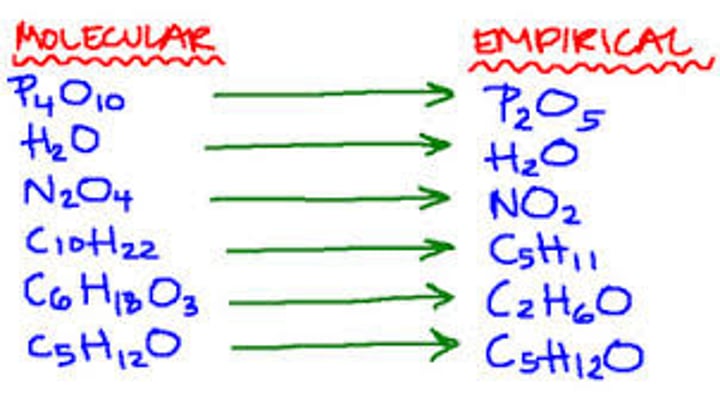
Molecular and Empirical Formula
Molecular formula show all of the atoms present in a molecule.
Empirical formula shows the nearest whole number ratio
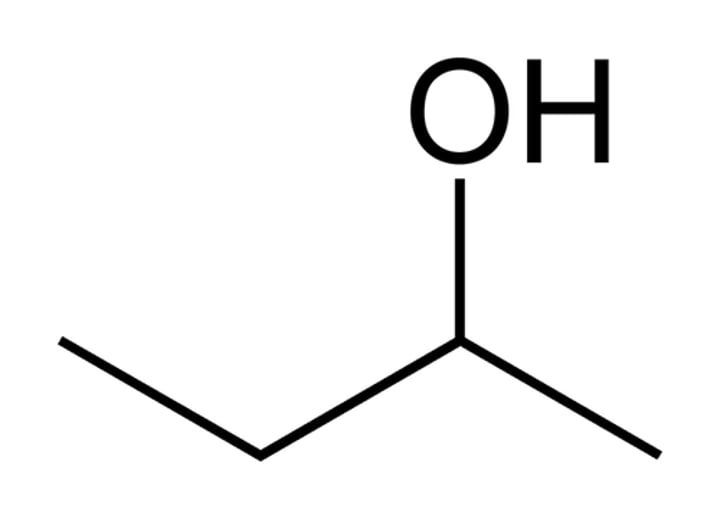
Structural formula
such as CH3CH2CH3 for propane
Skeletal formula
Each end of a line is a carbon atom. Any other compounds are added with a small line like the OH. Double bonds are shown by a double line.
Homologous series
Family of chemical with the same general formula and similar chemical properties
E.g. Alkenes are a homologous series with general formula CnH2n
Functional group
An atom or group of atoms that give the compound some distinctive and predictable properties
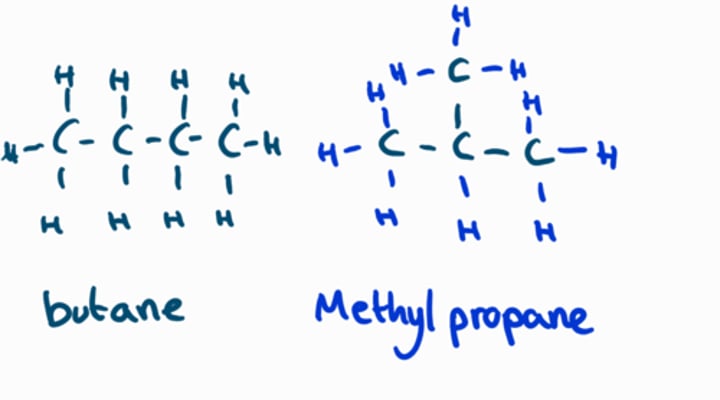
chain isomerism (structural)
refers to molecules with different carbon chains.
position isomerism
Same molecular formula functional group in different position on the same carbon chain.
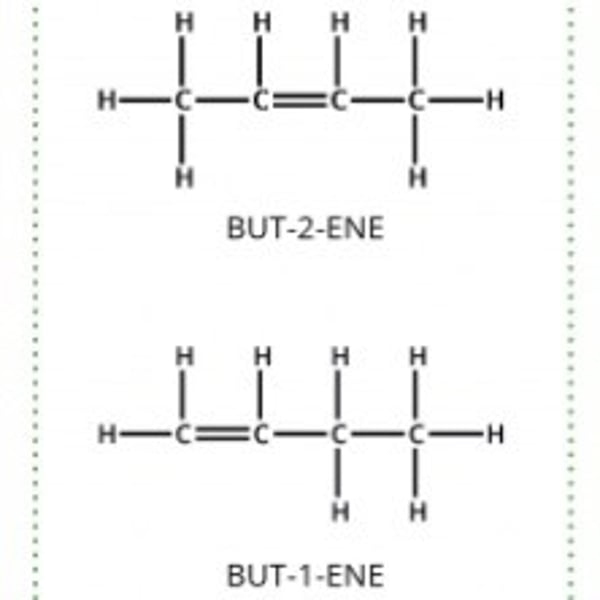
Sterioisomerism
Stereoisomers have:
The same molecular formula
The same structural formula
BUT different arrangements (e.g. geometric and optical isomers)
Geometric Isomers
Geometric isomers : compounds containing a C=C bond with atoms or groups attached at different positions.
These types of isomers rely on there being a double bond as that can't twist. trans is when the same compound is across the center from each other and cis is when they are on the same side.
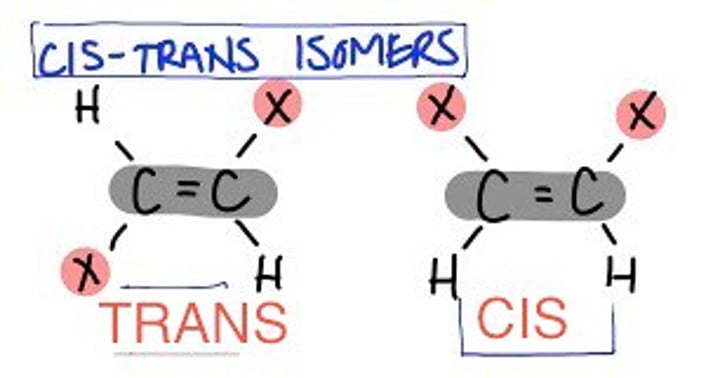
Trans-(E)
across, through
(Either Side)
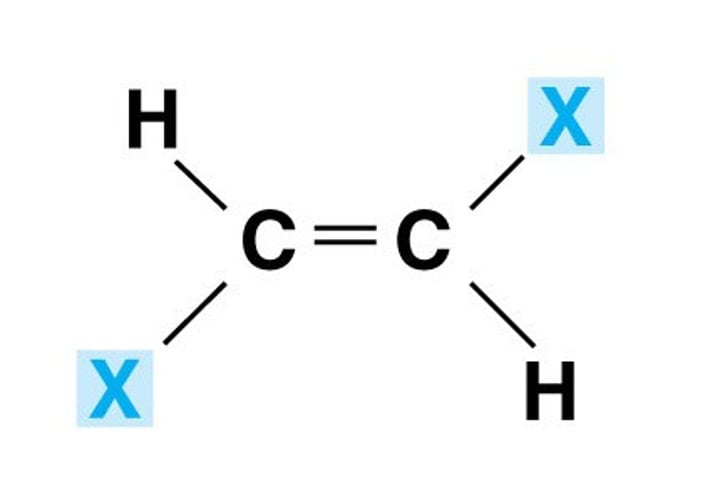
Cis-(Z)
Zame Zide
Fractional Distillation
- Crude oil is heated and vapourised
- Sent up a distillation column with a temperature gradient
- Different chain lengths condense at different fractions as they have different melting temperatures
-Dissolved gases just go straight through the column
Cracking
Long chain hydrocarbons with fewer uses undergo cracking to break it into a smaller alkene and alkane.
Cracking conditions
The long chain alkane is passed through a heated zeolite (Aluminium, silicon and oxygen) catalyst.
Reforming
The processing of straight-chain hydrocarbons into branched-chain alkanes and cyclic hydrocarbons for efficient combustion.
Reforming conditions
Heated with a platinum catalyst
Products of complete combustion of alkanes
Produces Carbon dioxide and water
Products of incomplete combustion of alkanes
Incomplete combustion occurs due to insufficient combustion or too rapid combustion. It produces:
- Solid carbon (soot)
- Carbon monoxide
Formation of Oxides of Sulphur
The Sulphur comes from impurities in hydrocarbons
S + O2 -> SO2 and
2SO2 + O2 -> 2SO3
These are acidic oxides so they dissolve in water in the atmosphere
SO2 + H2O -> H2SO3 and
SO3 + H2O -> H2SO3
Both cause acid rains which damages the environment
Formation of Oxides of Nitrogen
High temperatures especially around the spark plug cause N2 in the atmosphere react with O2
N2 + O2 -> 2NO
2NO + O2 -> 2NO2
These also dissolve in water in the atmosphere
2NO2 + H2O -> HNO2 + HNO3
Both of these acids damage the environment
Catalytic converters
Use a mesh covered in rhodium, platinum, and palladium catalysts to increase the rate of reaction (and make it cheaper).
They turn the pollutants NOx, CO and unburnt hydrocarbons into N2, H2O, CO2
2CO + O2 -> 2CO2
2NO + 2CO -> 2CO2 + N2
Unburnt hydrocarbons undergo complete combustion with oxygen
Sulphur is best removed before burning the fuel
Carbon neutrality
Where no net carbon is emitted
Biofuels
a fuel derived directly from living matter.
(though still not carbon neutral due to transport and processing)
Biodiesel
Biodeisel is made from vegetable oils (such as rapeseed of sunflower oil) and can be mixed with normal diesel as well
Biolchohols
Can be produced by the fermentation of sugar, but there's a limit to the ethanol concentration of fermentation, so the ethanol has to be separated from a lot of water (which takes energy) before it can be used.
Bioethanol is now produced with bacteria rather than enzymes, which has a higher yield of ethanol for a smaller starting amount.
Biodiesels vs Natural Gas: Biodiesel
Land use:
A lot of land is needed, this could be used to grow food
Yield:
Low but gradually increasing
Manufacture/Transport:
No exploration or drilling but a lot of growing, manufacturing and transportation costs
Carbon Neutrality:
Much closer to carbon neutrality
Biodiesels vs Natural Gas:
Natural Gas
Land use:
No land needed
Yield:
Very high
Manufacture/Transport:
Exploration and drilling costs high but processing and transport (via pipelines) low
Carbon Neutrality:
Definitely not carbon neutral
Radical
A species with an unpaired electron.
In mechanisms it is representated by a single dot. It is formed by homolytic fission (gives you two identical compounds) of a covalent bond.
Mechanism of radical substitution
Initiation:
UV light breaks apart Cl2 molecules to form Cl radicals
Propogation:
The Cl radicals are extremely reactive and react with the methane.
Termination:
If two radicals react to form a stable compound, it's a termination reaction
This can happen with halogens other than Cl2 (x in diagram) or methane (R in diagram)
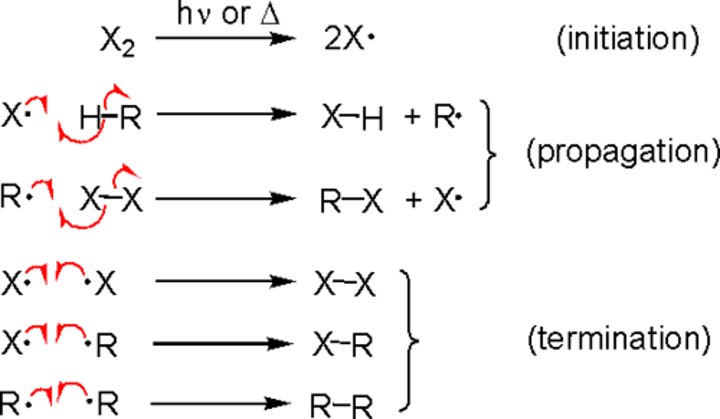
Products of Radical Substitution
Products have limited uses as a mixture of products is produced.
Further reactions are also hard to hinder:
CH3Cl + Cl2 -> CH2Cl2 + HCl
CH2Cl2 + Cl2 -> CHCl3 + HCl
CHCl3 + Cl2 -> CHCl4 + HCl
In this way unwanted dichloro, trichloro, and tetrachloromethane is formed
Alkenes
Alkenes are a homologous series of unsaturated hydrocarbons with a general formula of CnH2n (CnH2n-2 for cycloalkenes)
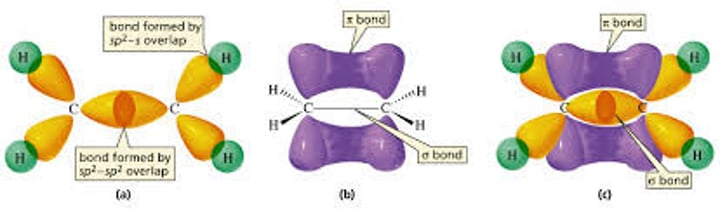
Bonding in double bonds
A double bond contains a sigma bond (when the end of two carbon orbitals overlap). The second bond however is a pi bond formed by the sideways overlap of two more orbitals.
These double bonds have a very high electron density
Electrophile
A species that is attracted to electrons
Addition reactions of Alkenes
-Hydrogenation
-Halogenation
-Hydration
-Addition of hydrogen halides
- Oxidation to diols
Hydrogenation
Hydrogen is added to an alkene in the presence of a nickel catalyst to produce an alkane
The hydrogenation of saturated vegetable oils is used to make margarine
Halogenation
Halogens are added to alkenes under no specific conditions to produce dihalogenoalkanes.
This is used to test whether a compound is an alkene or alkane as an alkene will react with Br in Bromine water to turn it colourless.
Hydration
Water (in the form of steam) is added to an alkene is the presence of Phosphoric acid (H3PO4) to produce alcohols
Addition of Hydrogen Halides
Hydrogen halides are added under no specific conditions and form a halogenoalkene
Oxidation to diols
Water and an oxygen from an the oxygen agent (depicted as [O]) Potassium manganate (VII) (KMnO4) is added to an alkene in acidic conditions to produce a diol
Hetrolytic fission
The hetrolytic fission of a covalent bond results in two ions being formed as one of the atoms takes the electrons
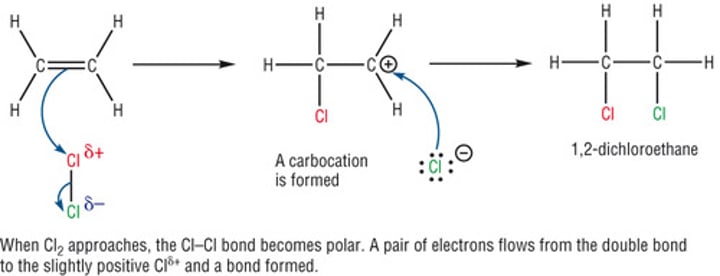
Mechanism of Electrophilic additions
As the Cl2 approaches the ethene, a temporary dipole is set up due to the high electron density of the double bond.
The Cl+ ion reacts with the ethene, opening the double bond and leaving a carbcation.
This then reacts with the Cl- ion
The same process can occur with hydrogen halides, but in that case the H already has a slight positive charge
Mechanism of Electrophilic additions:
Unsymmetrical molecules
In an unsymmetrical alkene, there are two possible products.
The Major product will always be formed from the more stable carbocation.
Primary, Secondary and Tertiary Carbocations
Primary carbocations are when the positive carbocation is only next to one other carbon, Secondary when it is next to two other carbons and tertiary when it is next to three.
As the carbons c=sharethe positive charge, secondary carbocations are more stable than primary ones, and tertiary are more stable than secondary
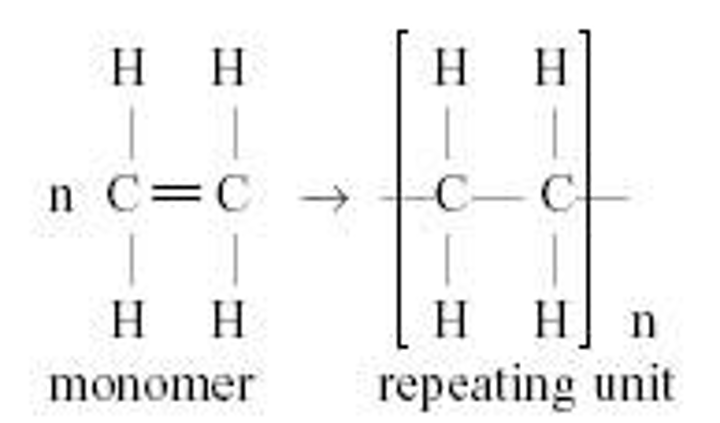
Polymer
A long molecule consisting of many similar or identical monomers linked together.
monomer
A simple compound whose molecules can join together to form polymers
Repeat unit
is the monomer that is repeated in a polymer. It is drawn with the bonds opened up
Addition reaction to form a Polymer
Dealing with waste polymers
-Recycling
-Incineration
-Chemical feedstock
Recycling
-First sort the polymers (done by hand and very slow)
-Next processing (chopping and then make it into a new material)
Incineration
Polymers are predominantly carbon and hydrogen and so burn well.
However, any other molecules such as Cl2 are also emitted into the air as well as toxic metals that were used to colour the polymers.
Chemists can try to remove the toxic waste gases, but they are difficult to remove.
Chemical Feedstock
Polymers can be broken down into waste gases (predominantly hydrogen and carbon) which can then be used for chemical processing such as making more polymers
Biodegradable polymers
Polymers can be manufactured to break down by microbes and the environment.
However this uses plant materials (which could be used for food)
Polymer lifecycle
Polymer life cycles can show the affect that polymers have on the environment at different stages of their lives
Types of Halogenoalkanes
There are primary, secondary and tertiary hydroalkanes
Reactivity of Halogenolakanes
The C-X bond is polar as halogens are very electronegative. The more electronegative the halogen, the more polarised the bond and therefore it is more susceptible to attack from a nucleophile.
A C-Cl bond has a higher bond enthalpy than a C-I bond and is thus harder to break.
Nucleophile
A species that donates a lone pair of electrons to form a covalent bond with an electron deficient atom
Halogenoalkane substitution reactions
RX -> ROH (add H2O and heat)
RX -> ROH (add KOH and heat under reflux)
RX -> RCN (add kCN and heat under reflux)
RX -> RNH2 (add NH3 and heat in a sealed tube)
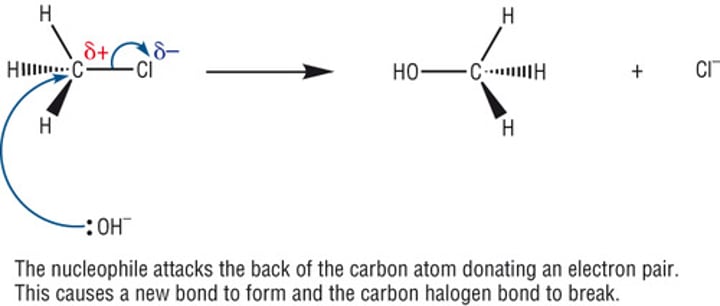
Hydrolysis of Halogenoalkanes
The δ- oxygen atom in water acts as a nucleophile and is attracted to the δ+ carbon atom next to the halogen.
The following reaction ensues:
RX + H2O -> ROH + HX
creating an alcohol
Experiment about hydrolysis of Halogenoalkanes
-Add silver nitrate as well so you can see how quickly the reaction occurs
-Use ethanol as a solvent (so that the alcohol dissolves)
- Control temperature, concentration and quantity of the halogenoalkane
-Time to the first appearance of the precipitate
Interpreting results for hydrolysis of halogenoalkanes
-The more electronegative the halogen, the more polar the C-X bond and thus the stronger the δ+ of the carbon atom, so the reaction occurs faster.
- Tertiary halogenoalkanes react faster than secondary halogenoalkanes, which react faster than primary halogenoalkanes
Potassium hydroxide and Halogenolaklanes
-The OH- ion is the attacking nucleophile
-The product is an alcohol
Potassium cyanide and Halogenoalkanes
- The CN- ion is the attcking nucleophile
- The product is a nitrile
-This extends the carbon chain by one, as CN has been added
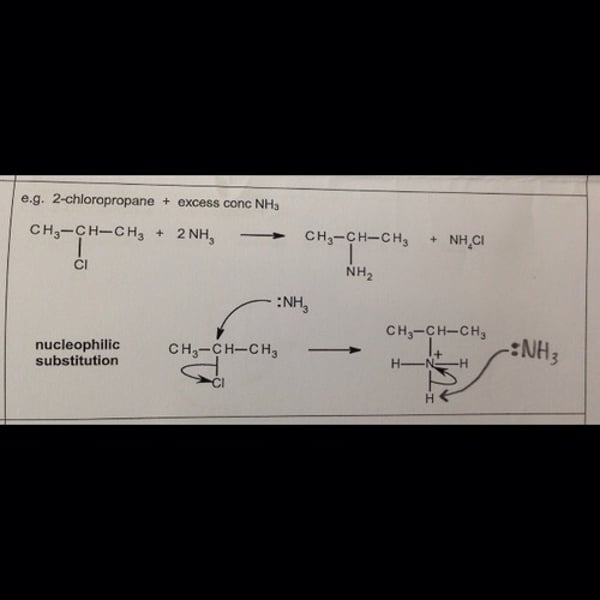
Ammonia solution and Halogenoalkanes
- The ammonia molecule (the lone electron pair on it) is the attacking nucleophile
- The product is a primary amine
- The NH3 attaches itself to the organic compound first, creating an ion (because the halogen has taken the electrons from the C-X bond) , then an H+ from this ion reacts another ammonia molecule to form an ammonium ion
Ethanolic potassium hydroxide and halogenoalkanes
If this reaction occurs with ethanol as a solvent rather than water, an alkene is produced rather than an alcohol
-The OH- acts as a base, not a nucleophile
-The halogen is removed to form KX
-A hydrogen from the carbon next to the one in the C-X bond reacts with the OH- (forming a double bond then) creating water
-So the products are KX, H2O and an alkene
-This is an elimination reaction
Mechanism of nucleophilic substitution OH-
Mechanism of nucleophilic substitution NH3
Alcohols
A homologous series of compounds with general formula CnH2n+1OH
They can be classified as primary secondary and tertiary as well
Combustion of Alcohols
Alcohols combust with oxygen in the air to produce carbon dioxide and water
Alcohols to Halogenoalkanes:
Chlorination
For Primary and Secondary Alcohols:
-Phosphorus(v) chloride is used (PCl5)
-Reaction is vigorous so no heating required
-Products are POCl3, HCl and a chloroalkane
Equation:
CH3CH2CH2OH + PCl5 -> CH3CH2CH2Cl + POCl3 + HCl
For Tertiary Alcohols:
-Alcohol is shaken at room temperature with concentrated HCl
-Products are chloroalkane and water
Equation:
(CH3)3COH + HCl -> (CH3)3CCl + H2O
Alcohols to Halogenoalkanes:
Bromination
First equation (inorganic products)
-Uses potassium bromide and 50% concentrated concentrated sulfuric acid
-Forms HBr and Potassium sulfate or potassium hydrogen sulfate.
KBr + H2SO4 -> KHSO4 + HBr
or
KBr + H2SO4 -> K2SO4 + HBr
Second equation:
-CH3CH2OH + HBr -> CH3CH2Br + H2O
Alcohols to Halogenoalkanes:
Iodination
-Uses red phosphorous and iodine
-The reaction mixture is heated under reflux
Inorganic Equation:
2P + 3I2 -> 2PI3
Organic Equation:
3C2H5OH + PI3 -> 3C2H5I + H3PO3
The inorganic product is phosphonic acid
Dehydrating alcohols to alkenes
-This is an elimination reaction
-The alcohol is heated with concentrated phosphoric acid
-The OH and a hydrogen from an adjacent carbon atom form water and leave behind an alkene
-This means that there can be more than one product (also watch out for E-Z isomerism giving you yet another product)
-The water formed dilutes the phosphoric acid
Oxidising Primary Alcohols
-Potassium (or sodium) dichromate(VI) is used as an oxidising agent
-Primary alcohols immediately oxidise to form aldehydes (with one carbonyl group at the end) there is a colour change from orange to dark green
-When heated under reflux, the aldehyde rapidly oxidises to form a carboxylic acid
-In reactions the oxidising agent is shown as [O]
First Equation:
CH3CH2OH + [O] -> CH3CHO + H2O
Second Equation:
CH3CHO + [o] -> CH3COOH
Oxidising Secondary alcohols
-Secondary alcohols oxidise to form ketones, a carbon chain with a carbonyl group in the middle
CH3CH(OH)CH3 + [O] -> CH3COCH3 + H2O
Oxidising Tertiary alcohols
Tertiary alcohols don't oxidise
Fehling's Solution
If a few drops of an aldehyde is added to Fehling's solution and then gently warmed, the blue solution will produce a dark red precipitate
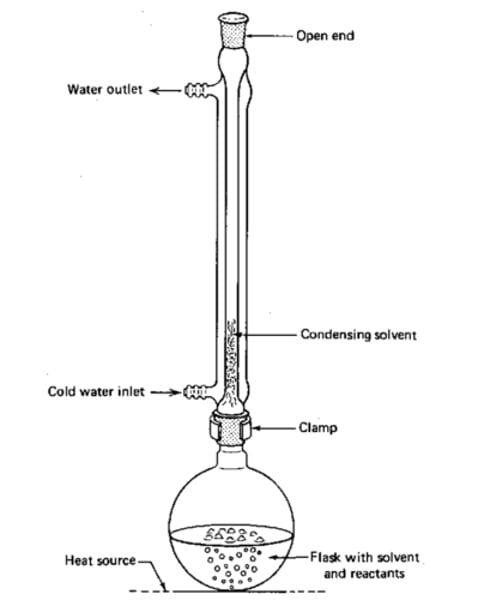
Heating under reflux
When oxidation is intended to be complete (for a ketone or carboxylic acid) the reactant mixture is heated under reflux
Distillation with addition
(Look up apparatus in book)
Is used to obtain an aldehyde as the alcohol is slowly added and as soon as the aldehyde forms it immediately distills off
Organic vs inorganic apparatus
-For inorganic compounds, glassware with corks, bungs and tubing are used
-Organic compounds are flammable, sometimes toxic and can attack corks and bungs
-So for organic compounds, chemists use equipment made predominantly of glass with tight fitting ground glass joints.
Simple Distillation
-Heat the impure liquid in a flask connected to a condenser
-The liquid with the lowest boiling temperature evaporates and condenses first
-A thermometer is used as if the temperature is steady, that is an indication that one liquid is distilling over. When the temperature increases again, it has all distilled over
Advantages:
Easier to set up and quicker than fractional distillation
Disadvantages:
It doesn't separate liquids as well as fractional distillation, it should only be used if the difference in boiling temperatures is larger than 25
Fractional Distillation
-Same apparatus as simple distillation but has a fractionating column as well
-The column is filled with glass beads where liquis can condense again.
-the water vapour undergoes multiple distillations and so the liquids are separated more effectively
Solvent extraction with a separating funnel
-A solvent that is immiscable with the solvent containing the organic product and in which the organic product is much more soluble is added
-Place the reaction mixture and the solvent in a separating funnel
-Place the stopper on the neck and shake gently
-Allow two layers to form
-Remove the stopper and open the tap to allow the bottom liquid to drain out.
However, now the product must be removed from the new solvent
Drying
-The drying agent (an anhydrous salt) is added to the mixture, swirled, shaken then left
-The drying agent turns from powdery to crystaline
-If more drying agent remains powdery the liquid is dry
-the drying agent is removed by decantation or filtration
Testing for purity
-Measure the boiling temperature as impurities increase the boiling temperature
-The apparatus for a simple distillation can be used