Topic 11: Nuclear Radiation - Model Answers
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
53 Terms

In this nuclear symbol shown, state what is meant by the numbers 238 and 92.
238 is the nucleon number (mass number). This is the total number protons and neutrons in the nucleus of the atom.
92 in the proton number (atomic number). This is the number of protons in the nucleus. This defines which element is present.
State what is meant by the term ‘isotope’.
Isotopes are atoms that have the same number of protons in the nucelus as each eachother (and are the same element), but different number of neutrons (so different nucleon numbers).
Define the term ‘nucleon’.
A proton or a neutron - a particle within the nucleus.
Define nuclear binding energy.
The energy released when a nucleus is formed from its consituent nucleons OR the energy required to split a nucleus into its consituent nucleons.
Due to the mass deficit and ∆E = ∆mc².
Explain why there is energy released when a nucleus is formed from its consituent nucleons.
The total mass of all nucleons (protons and neutrons), is higher than the mass of the resulting nucleus.
So there is a mass deficit when a nucleus is formed, ∆m.
So there is energy released due to ∆E = ∆mc²
Define mass deficit.
The total difference in mass between the reactants and products in a nuclear reaction.
Define the atomic mass unit.
1/12 of the mass fo a single carbon atom, u = 1.66 ×10-27 kg.
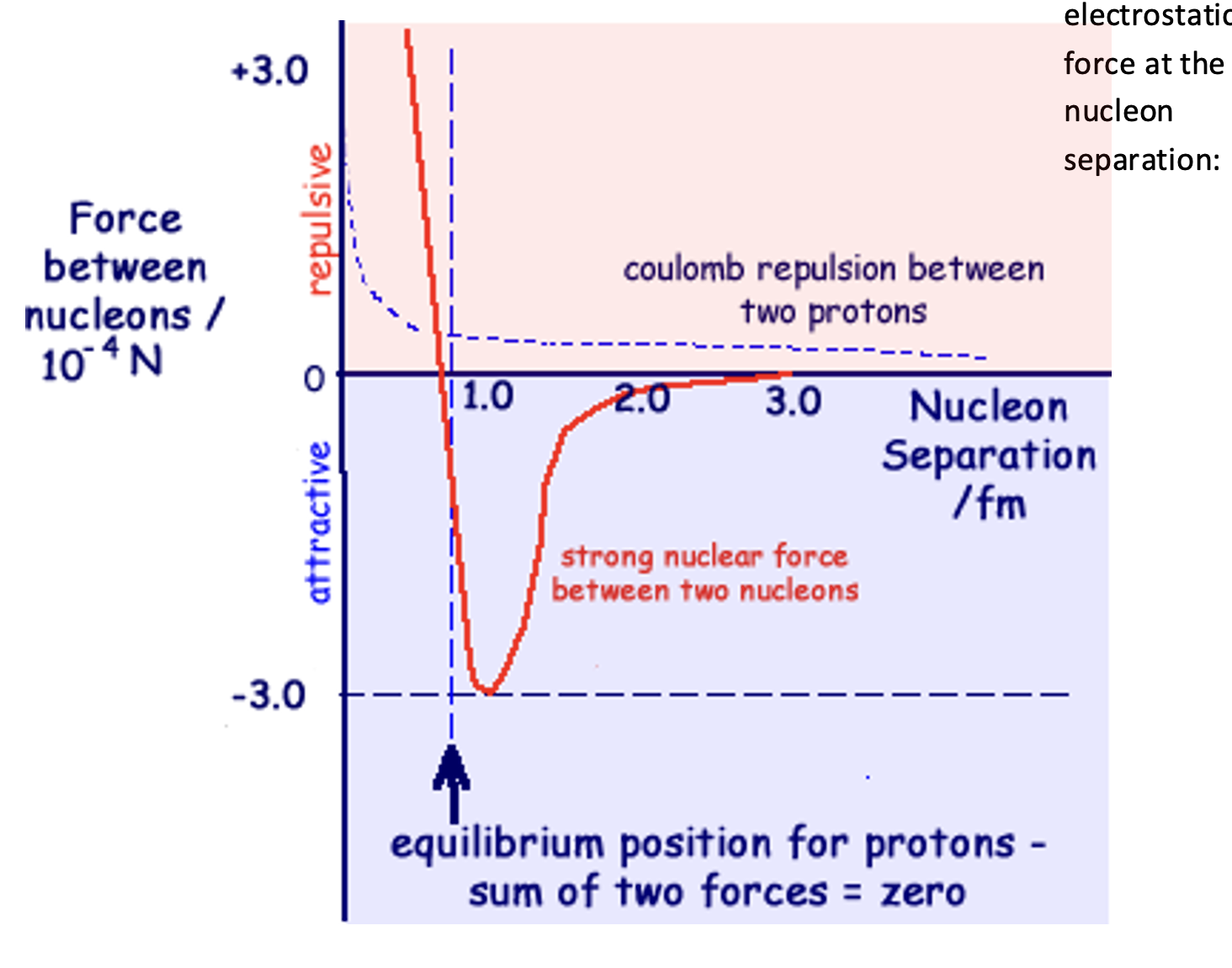
Describe what the strong force is and identify when it acts.
The strong force is the very strong force that acts between quarks.
It is repulsive at extremely low distances, but attracting at very low distances.
The force is responsible for ensuring the nucleons in the nucleus are held tightly together, balancing the electrostatic force at the nucleon separation.
Describe what is meant by nuclear fusion.
When small nuclei come close enough together for the strong force to act.
Allowing them to join to form a more massive nuceleus
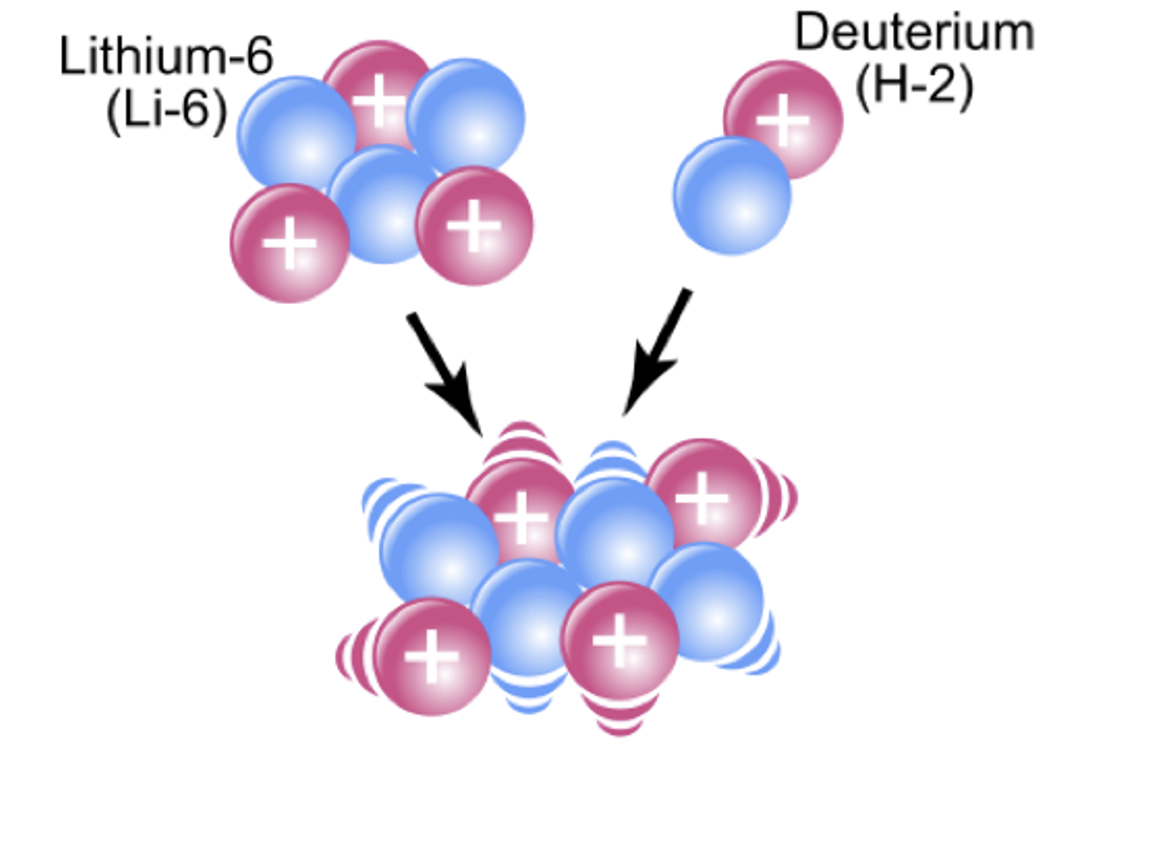
Explain why the fusion of hydrogen nuclei releases energy.
The mass of the fused nucleus is less than the total mass of the fusing nuclei.
There is therefore a mass deficit which releases energy due to ∆E = ∆mc2.
The binding energy per nucleon of the fused nucleus is greater than the constituent nuclei (and as binding energy per nucleon is the energy released per nucleon when a nucleus is formed, if this increases, energy must be released).
Describe what happen during nuclear fission.
Massive unstable nuclei (e.g. Uranium) split into two less massive daughter nuclei, releasing energy,
Describe what is meant by induced nuclear fission.
When slow moving neutrons are absorbed by massive nuclei.
Causing them to split into two less massive daughter nuclei.
and 2 or 3 additional neutrons.
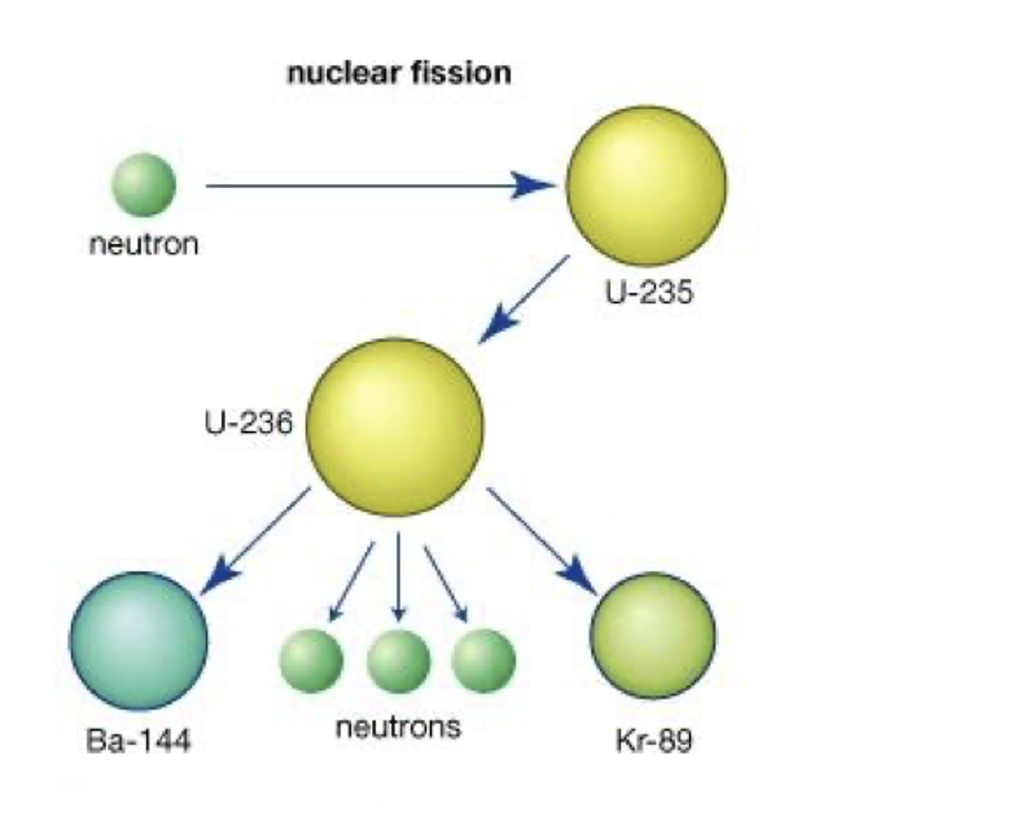
Describe how a chain reaction can occur in a nuclear fission reactor.
Each nuclear fission reaction produces 2 or 3 more neutrons.
These neutrons can be absorbed by 2 or 3 mofe uranium nuclei.
Resulting in 2 or 3 more fission reactions.
So one reaction causes 2 or 3 more.
This process continues and this is a chain reaction.
Sketch a graph showing the binding energy per nucleon for different nucleon numbers adn identity where fission and fusion occur and the element with the highest energy per nucleon.
Iron has the highest binding energy per nucleon - stable, no fusion or fission.
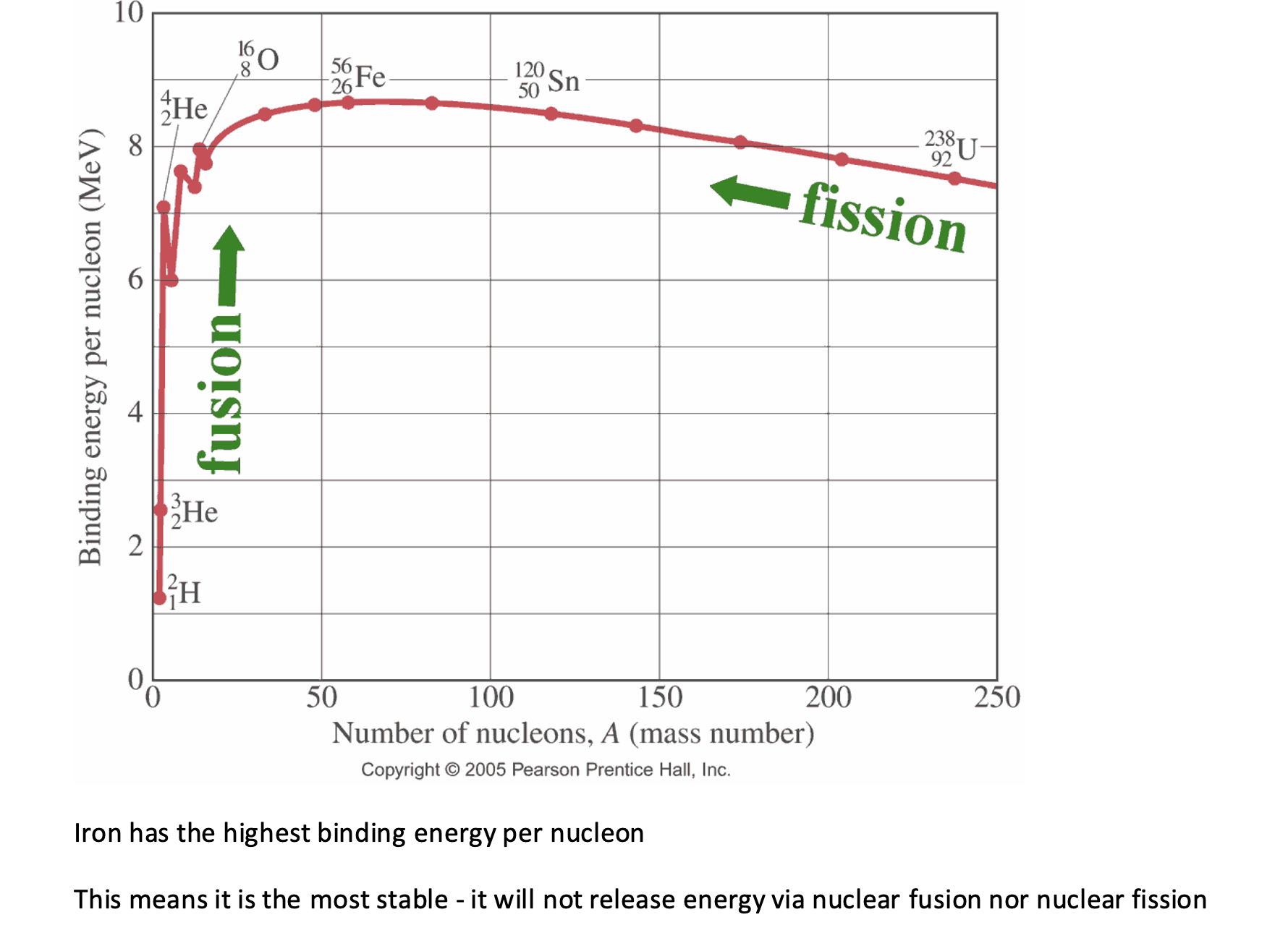
Explain, with reference to binding energy, why both fission and fusion release energy.
In fusion, the nucleon number increases when small nuclei join together, resulting in an increase in binding energy per nucleon.
In fission, the nucleon number decreases when large nuclei split apart, resulting an increase in binding energy per nucleon.
As the binding energy per nucleon is the energy released when a nucleon forms from its constituent nucleons, if this increases, energy is released.
Explain the conditions required for nuclear fusion to be maintained.
Extremely high densities are required to maintain a sufficient collision rate.
Extremely high temperatures are required to enable the nuclei to have enough kinetic energy to: overcome the electrostatic repulsive force and to come close enough together for the strong nuclear forces to act.
Explain why it is difficult to maintain the consitions needed for fusion within a nuclear reactor.
Extremely high temperature plasma is required for fusion.
If plasma touches the walls of the reactor, its temperature would drop (as it would transfer energy to the walls).
So very strong magnetic fields must be used to control the plasma to prevent it from touching the walls.
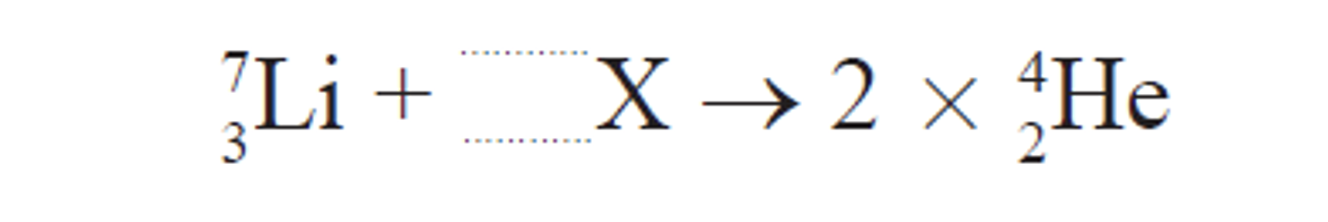
Complete the fusion reaction and identify X.
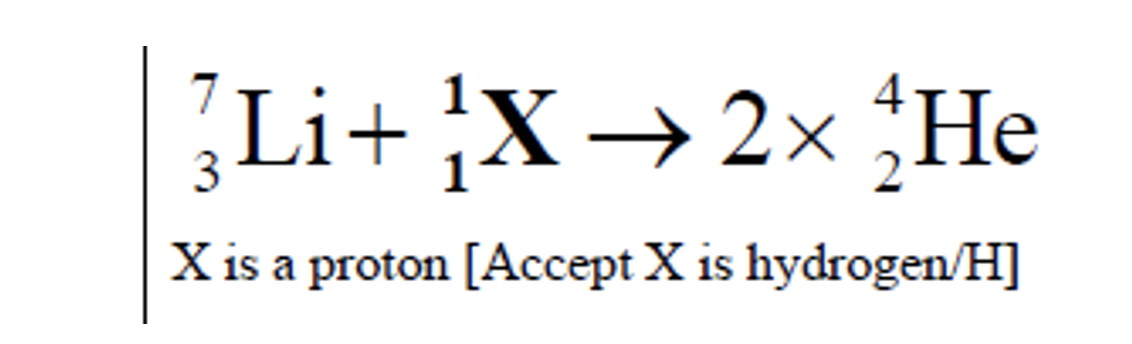
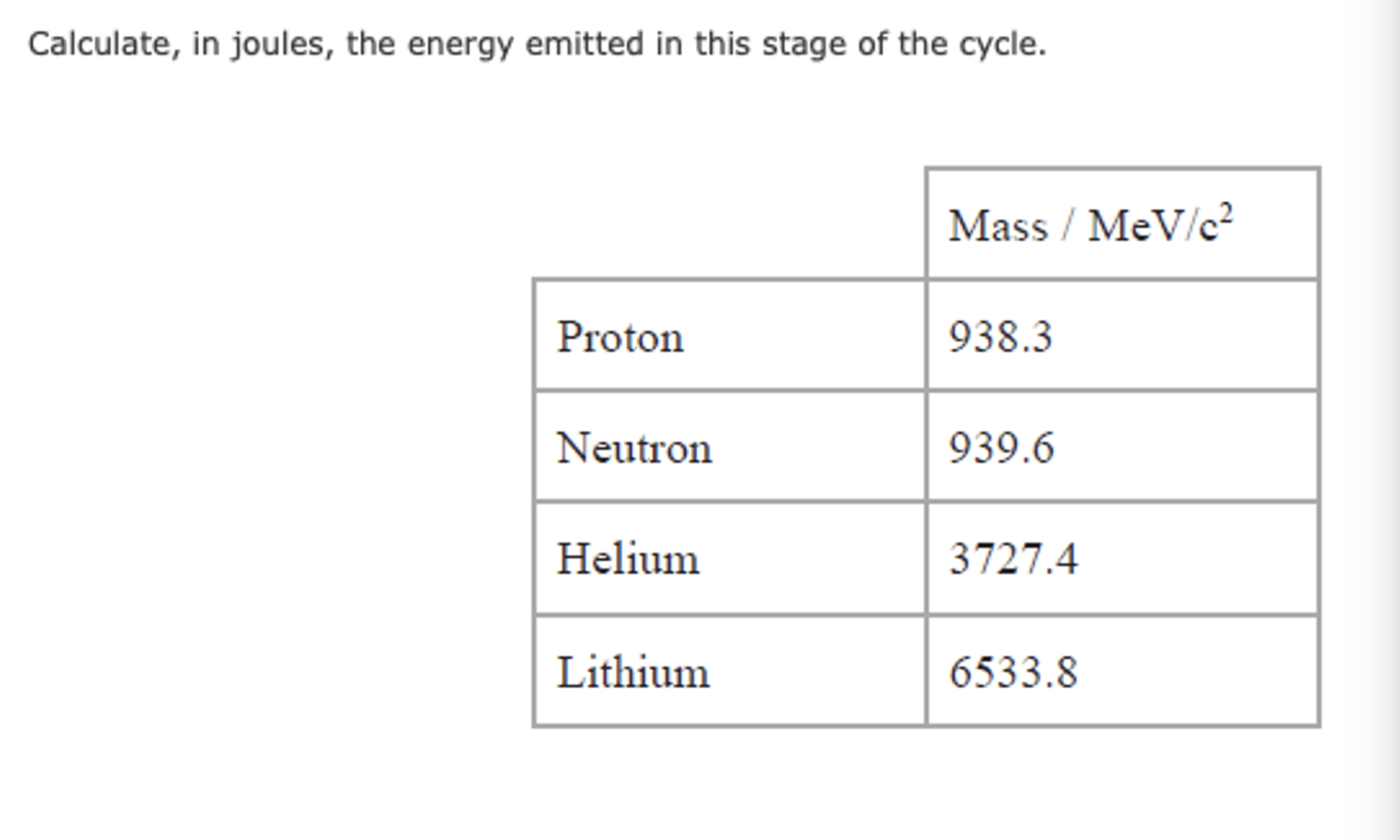
Mass deficit and energy release questions example 1.
Calculate mass deficit (mass of reactants - products), in the unit provided.
Calculate ∆E = ∆mc2.
Example one: mass unit is MeV/c².
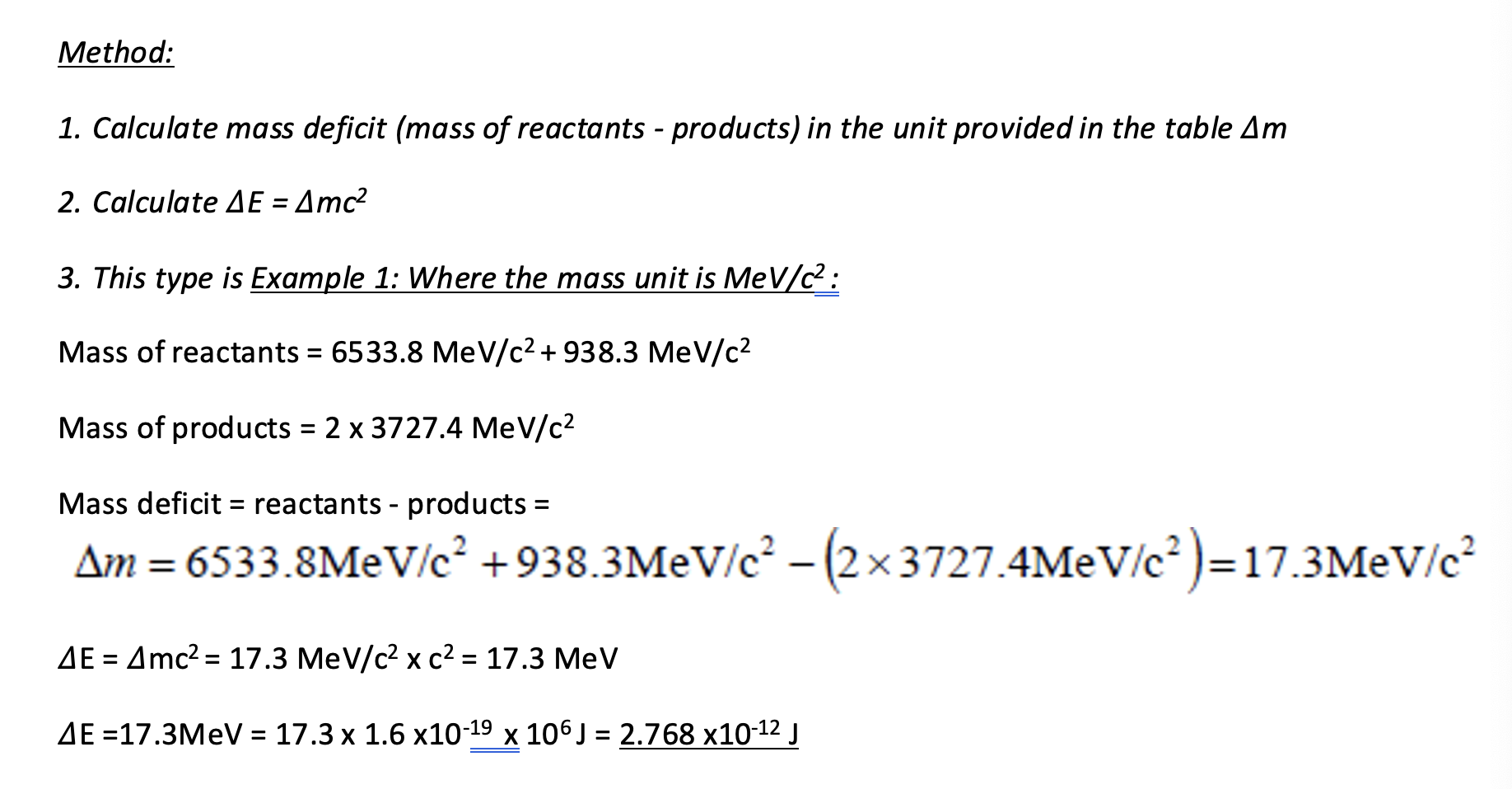
Mass deficit and energy release questions example 2.
Calculate mass deficit (mass of reactants - products), in the unit provided.
Calculate ∆E = ∆mc2.
Example one: mass unit is kg.
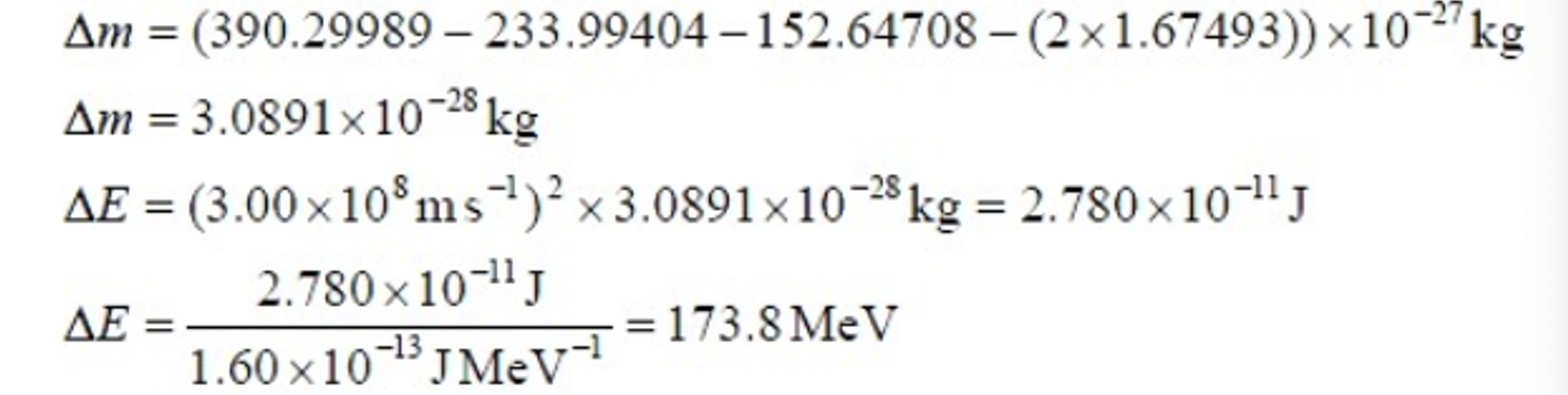
Explain why nuclear fission reactors can release large amounts of energy.
Even though each nuuclear reaction releases small amounts of energy,
The collision rate can be very high
And in a nuclear fission reactor, a chain reaction can be set up, which will mean the total energy released can be high.
Describe what happens during radioactive decay.
An unstable nucleus spontaneously and randomly decays into another nucleus, releasing alpha, beta or gamme radiation.
This process occurs with a mass deficit ∆m - the starting nuclei have a higher total mass than the products and the binding energy per nucleon increases.
So energy is released according to ∆E = ∆mc2
The energy is carried away by the products in their kinetic energy stores.
State the difference between the following terms: ionising radiation, radioactive source, unstable nucleus, unstable isotops.
Ionising radiation - alpha, beta or gamma radiation (particles/ radiation capable of removing electrons from atooms to form ions)
Radioactive source/ unstable nucleus/ unstable isotope - all of these terms refer to the nucleus that releases the ionising radiation during radioactive decay to become more stable.
Explain why an alpha particle should not be described as radioactive.
Alpha particles do not undergo radioactive decay themselves.
They are the ionising radiation that is released by an unstable nucleus (the radioactive particle), during radioactive decay.
Give examples of the sources of background radiation.
Radon gas - released from rocks.
Artificial sources - e.g. Nuclear weapons testing.
Cosmic rays - enter the Earth’s atmosphere from space.
Rocks containing naturally ocuuring radioactive isotopes.
Explain how background radiation should be accounted for when taking measurements of radioactive substances.
First remove all additional sources and measure the background count using a GM tube and counter in a given time (e.g. 100s).
Divide the count by the time measured for to determine the count rate.
Repeat and average
To determine the count rate due to a specific source, measure the total count in a given time and calculate the count rate as above, then use the equation:
corrected count = total count rate - background count rate.
Describe the nature, range and ionising ability of alpha, beta and gamma radiation and state what they are absorbed by.
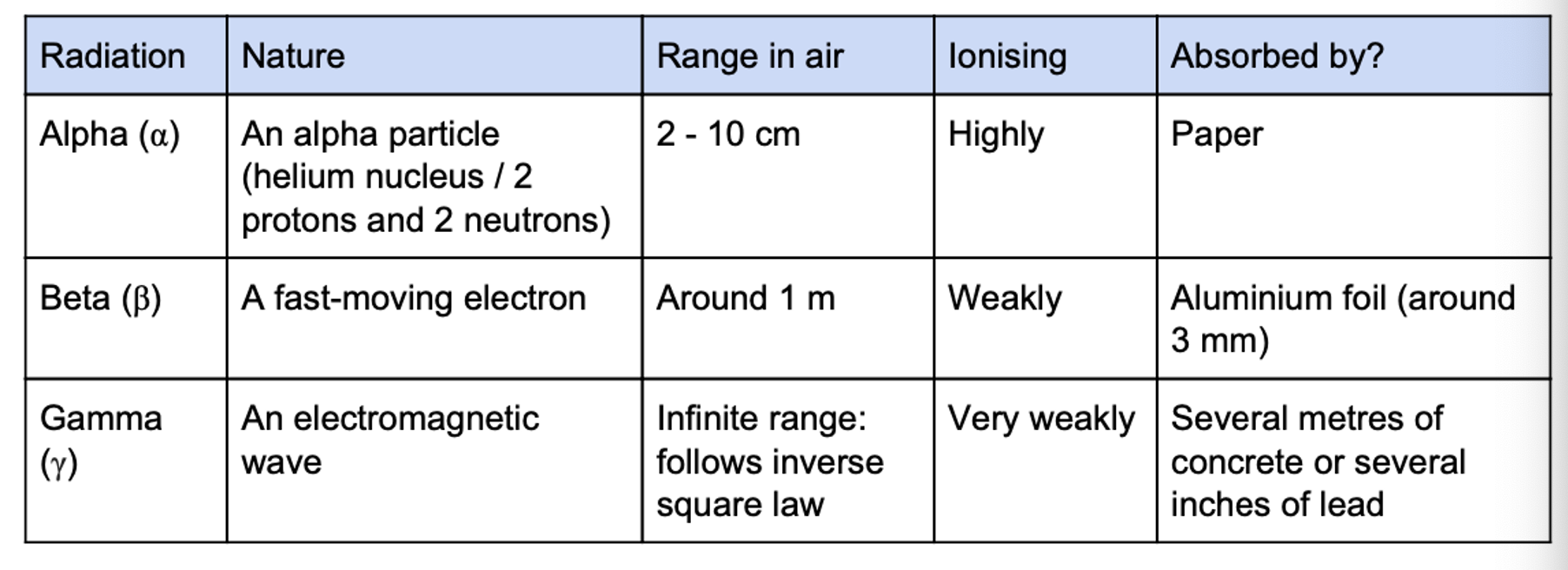
Describe an experiment to determine if a source is alpha, beta or gamma.
Use a GM tube and counter to determine the backgound count in 100s. Repeat and average.
Place the source close to the GM tube and counter adn determine the count in the same time - 100s. Repeat and average.
Place a sheet of paper between teh GM tube and counter. If the count in 100s decreases to the background level, the source is emitting alpha radiation.
Repeat the above process with a piece of aluminium foil. a few mm thick. If the count in 100 s decreases, the source is beta. If the count in 100s deos not decrease, the source is gamma.
Repeat the above process with several inches of lead, In the count in 100s decreases to background, the source is eimtting beta radiation.
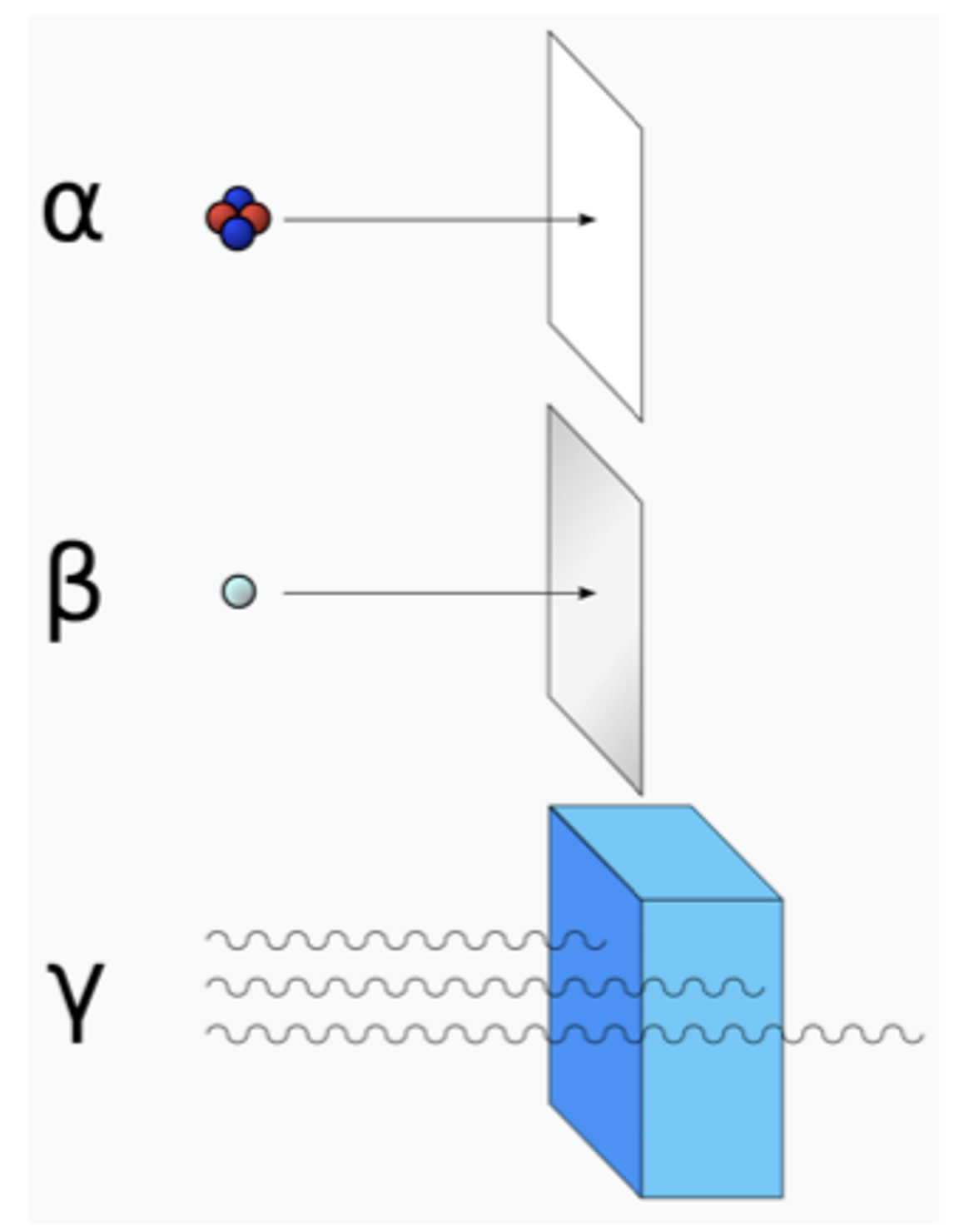
Explain a safety precaution for the above procedure.
Ensure the source is always pointed away from you and that there is at least 30cm between the source and the body, to minimise exposure.
Use tongs to handle the source to minimise exposure.
Because the source emits ionising radiation which can damage the DNA in your cells.
Explain why alpha radiation is the least penetrating.
Alpha radiation is most highly charged and most massive.
Therefore it is the most ionising, so it transfers all of its energy over shorter ranges than beta and gamma, and therefore has the lowest penetration power.
Explain why alpha sources are only dangerous if they enter the body.
Ionising radiation can be dangerous as it cuases damage to DNA in cells caused by ionisition.
But alpha particles cannot penetrate the dead layer of skin from outside of the body and therefore would not be able to cuase this damage.
Explain how a stream of alpha particles moving through the air can cuase an electric current between two parallel charged plates.
Alpha particles ionise the air, stripping electrons from air molecules.
The resulting ions and free electrons move within the electric field.
In a smoke detector, alpha particles are released and cause a current to flow as deccribed in the previous question. When smoke particles are present, the current decreases. Explain how.
Alpha particles collisde with the smoke particles and reduce the amount of ionisation. OR Smoke particles capture electrons and reduce the free charge able to move.
State the nuclear symbol for the beta particle.
(Beta has a charge of -1)
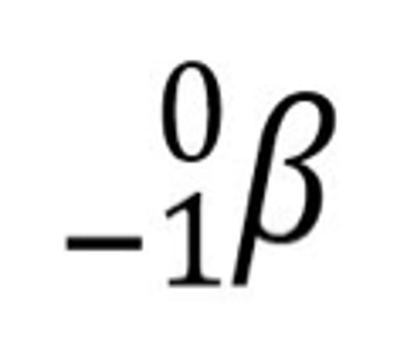
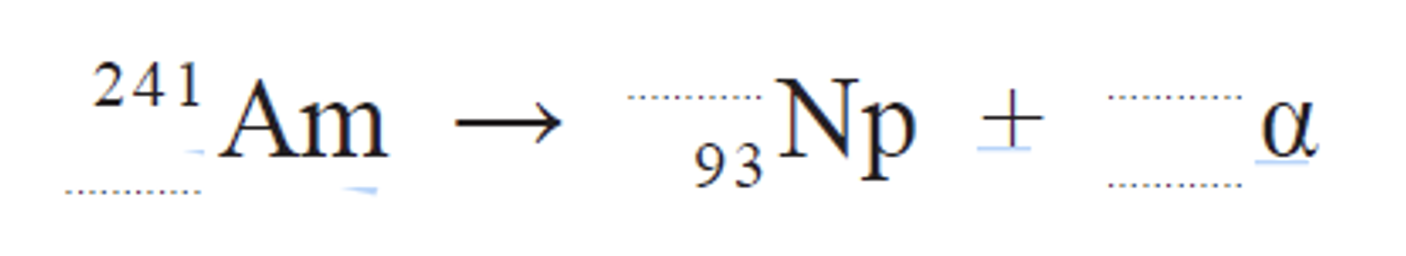
Complete the nuclear equation.
(4 alpha 2)
Explain how to increase the accuracy of the determination of count rate.
Increase the length of time for which count is measured.
This will reduce the % uncertainty in the measurement.
Repeat the measurement of count three times and take an average.
To reduce the effect of random error in the determination of count rate.
Remove background count.
As this would incur a systematic error.
Explain how to increase the accuracy of the determination of count rate of a gramma source specifically.
GM tubes have low sensitivities to gamma radiation (as gamma is highly penetrating and has a low ionising power).
So the GM tube should be orientated ‘side on’ to the source to increase the surface area exposed to the source and increase the count.
Define the term random in the conetext of nuclear decay.
Random means we cannot identify which nucleus will be the next to decay/when an individual nucleus will decay/ how many nuclei will decay in a set time/ can only estimate the fraction that will decau in the next time interval.
Define the term spontaneous in the context of nuclear decay.
Spontaneous means that the decay cannot be influenced by any external factors, e.g. temperature does not affect the rate of radioactive decay.
Define half life.
The average time taken for half of the unstable nuclei within a sample to undergo radioactive decay.
Define activity.
The number of unstable nuclei that decay per second.
A = -∆N / ∆t
Where N is the number of unstable nuclei remaining. The negative sign is present as the number of unstable nuclei decreases over time, making ∆N negative, so to make activity positive, we need the negative sign.
Describe the difference between count rate and activity.
Count rate is the number of decays per second detected by a GM tube, activity is the rate of decays in all directions.
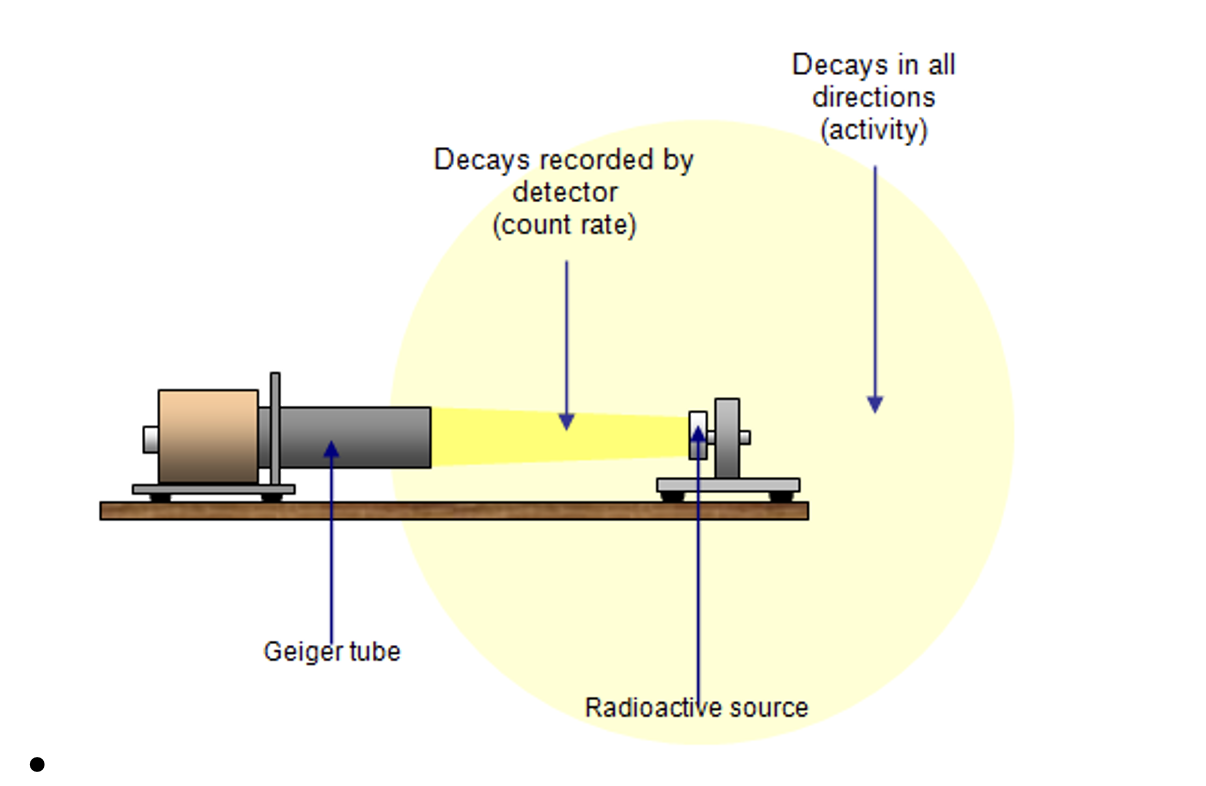
State and explain the equation for activity.
Activity, A is proportional to the number od unstable nuclei remaining, N (the more unstable nuclei present, the higher the rate of decay).
A = λN where λ is the decay constant.
As activity and number of unstable nuclei are proportional, the time taken for the activity to halve is also the half life.
Define what is meant by ‘the decrease in the number of unstable nuclei is exponential’.
This means that the ratio of the number of unstable nuclei to the initial number, N / N0 is fixed, for a fixed value of time.
So N = N0e-λt
Derive the equation for half life.
N = N0e-λt
Half life t1/2 is the time taken for the number of unstable nuclei remaining to halve.
So at t = t1/2, N = N0 /2
…

Polonium-210 has a half life of 138 days. In a 0.89 μg sample of polonium-210, there are 2.54 × 1015 atoms of polonium. Calculate the activity of a sample of this size.
A = λN. Activity is in Bq (units).

Polonium-210 has a half life of 138 days. In a 0.89 μg sample of polonium-210, there are 2.54 × 1015 atoms of polonium. Calculate the fraction of polonium-210 nuclei that have decayed after a time of 21 days.
t = 21 × 24 × 3600 = 1 814 400s
(Remember formula gives the fraction remaining, so fraction decayed = 1 - fraction remaining).

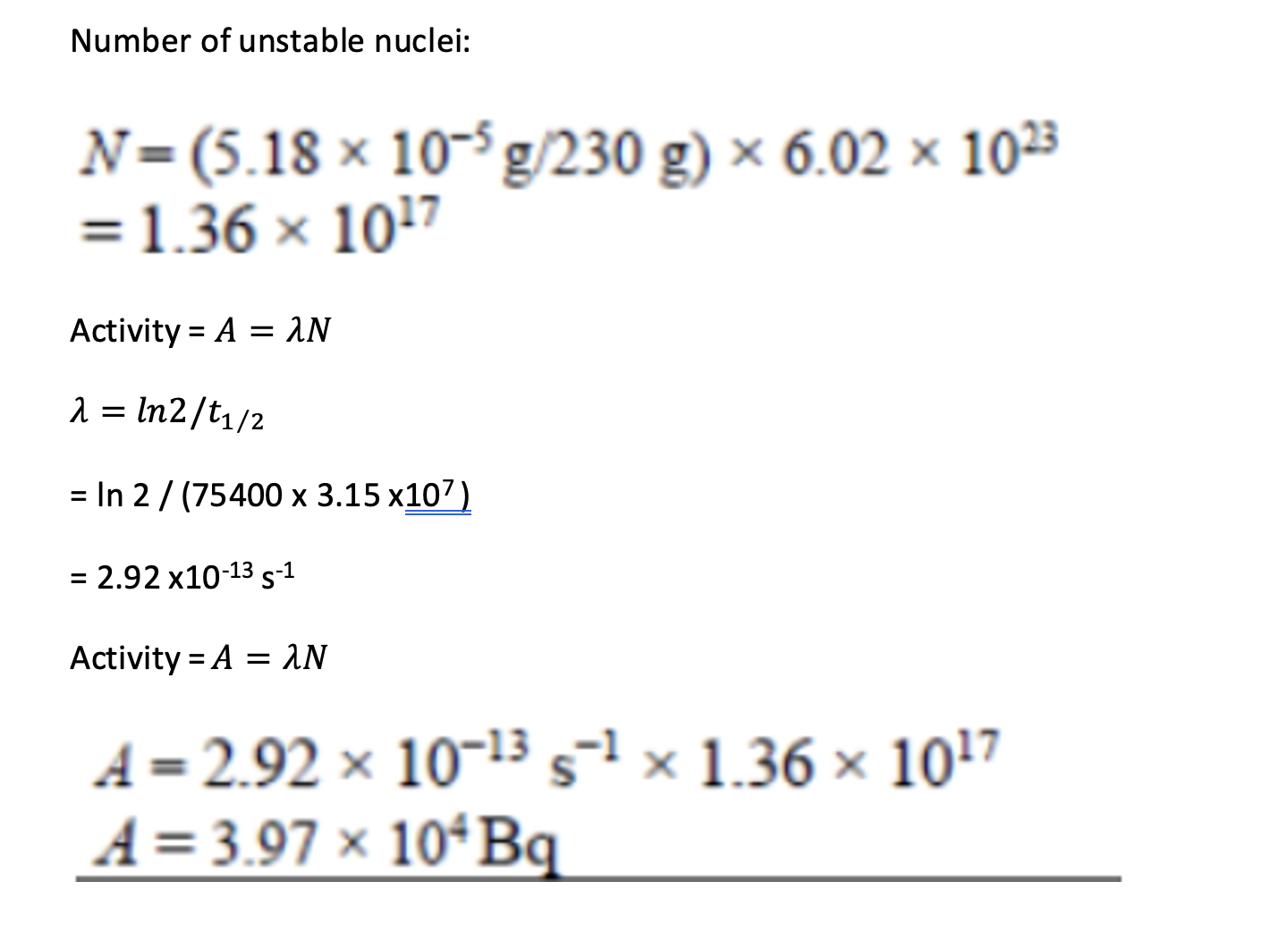
A particular gas mantle contains 5.18 × 10-5g of thorium-230. Show that the activity of the thorium-230 in the mantle is about 4.0 × 104 Bq. Data: 230g of thorium-230 contains 6.02 × 1023 atoms, half life is 75400 years, number of seconds in 1 year = 3.15 × 107.
… For every 103 uranium nuclei present at the start, 50 are now lead nuclei. Show that the age of the sample is about 4 × 109 year.
Find the number of nuclei at the beginning and the number of nuclei remaining : 103 - 50 = 53
(There is no need to change to seconds unless you have been asked to).

Radioactive isotopes are often used as markers, so that chemical substances can be traced around the body. In one medical procedure tritium is used as a means of studying protein absorption by the intestine. A patient was given a sample containing the tritium to drink and then monitored. The initial activity of the sample was 3450 Bq. Tritium is a beta-emitter with a half-life of 3.89 × 108 s.
i) Calculate the number of tritius nuclei in the initial sample.
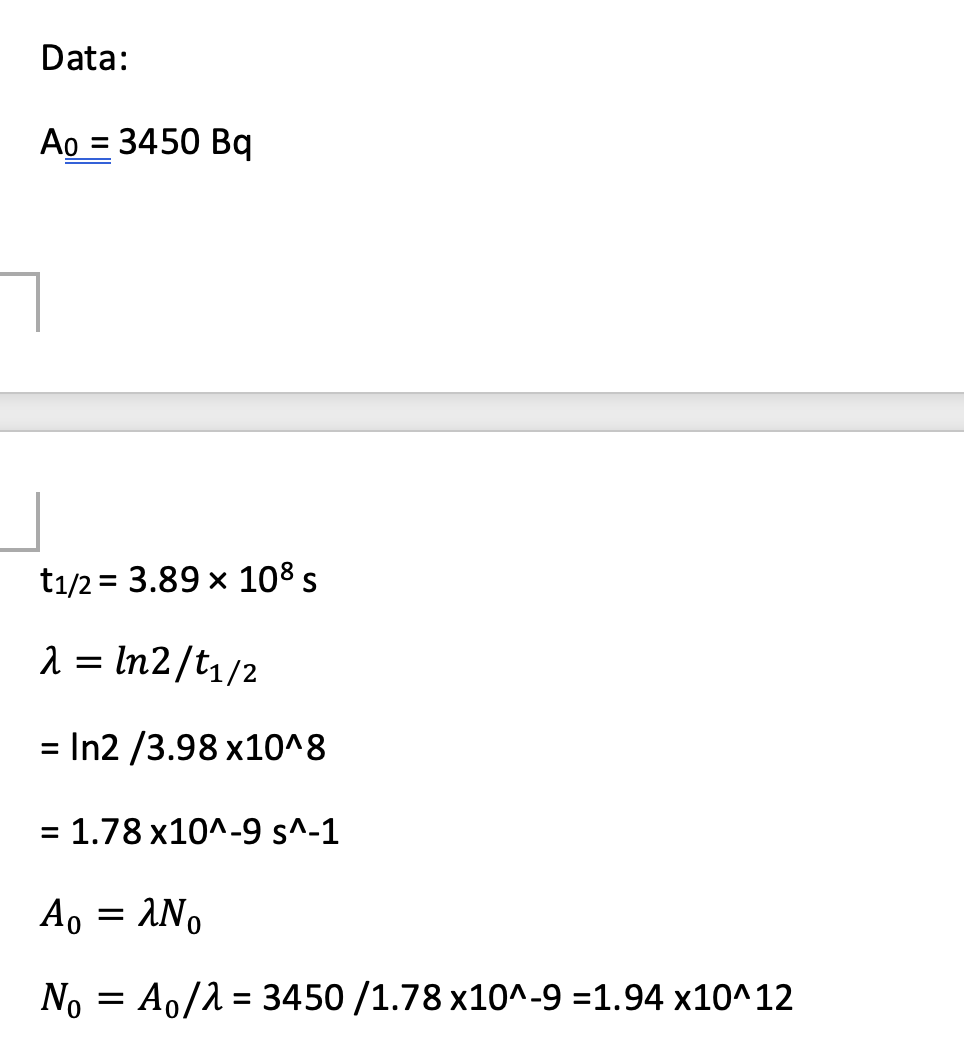
Radioactive isotopes are often used as markers, so that chemical substances can be traced around the body. In one medical procedure tritium is used as a means of studying protein absorption by the intestine. A patient was given a sample containing the tritium to drink and then monitored. The initial activity of the sample was 3450 Bq. Tritium is a beta-emitter with a half-life of 3.89 × 108 s.
ii) Show that the time taken for the activity of the sample to fall to 10% of its initial value is about 40 years.
Sample activity falls to 10% of original when A = 0.1A0
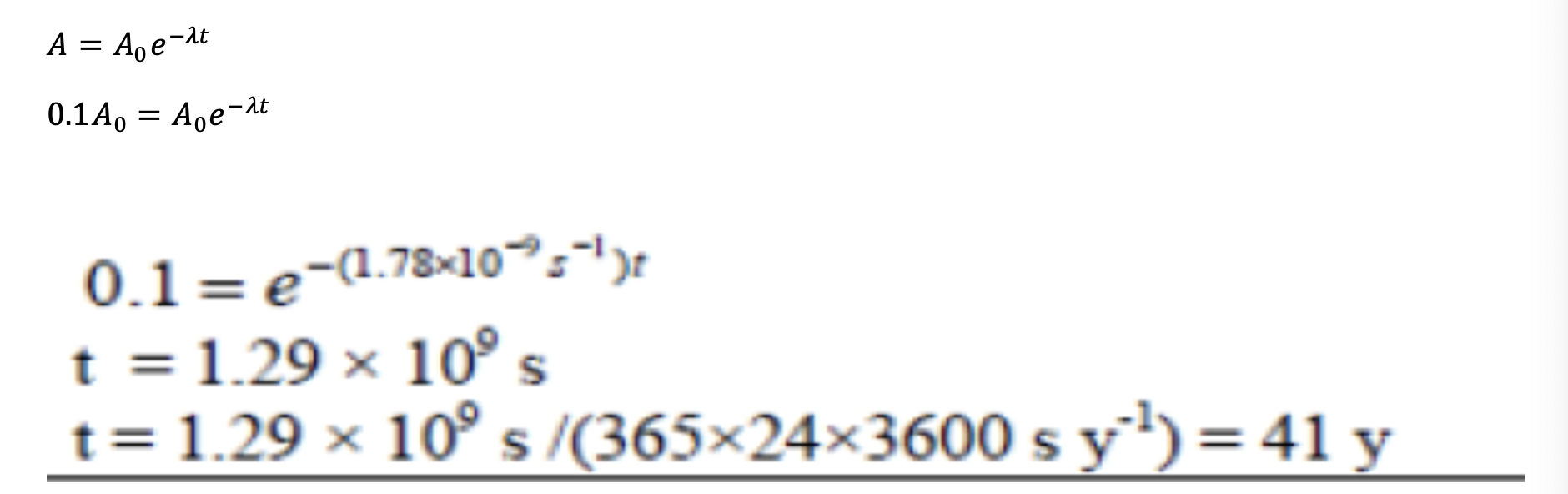
Radioactive isotopes are often used as markers, so that chemical substances can be traced around the body. In one medical procedure tritium is used as a means of studying protein absorption by the intestine. A patient was given a sample containing the tritium to drink and then monitored. The initial activity of the sample was 3450 Bq. Tritium is a beta-emitter with a half-life of 3.89 × 108 s.
iii) Comment of the time given in ii) (41 years)
This is a very long time compared to the length of time they would likely be monitored for so:
The sample’s activity will stay approximately constant throughout.
And the sample could be prepared long in advance of the procedure.
However, unstable tritium nuclei would remain in the body long enough for damage to potentially be caused.

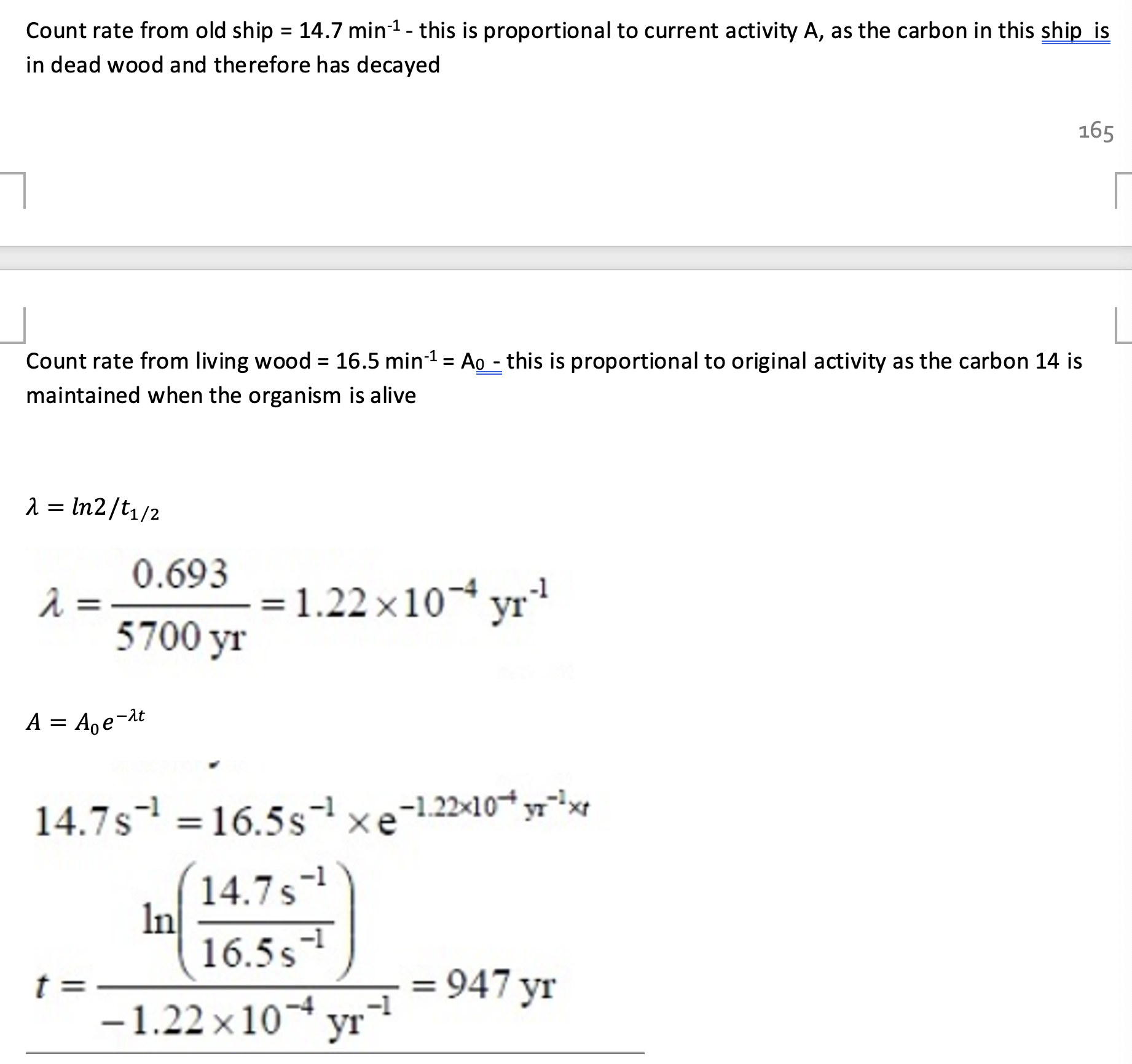
i) Calculate the age of the ship in years.
ii) The concentration of 14C in living organisms might have been greater in the past. Explain how this would affect the age that you have calculated.
Count rate is proportional to current activity.
Count rate from living wood is proportional to initial activity.
ii) The initial value for the count rate should be larger than 16.5 min-1, so we have underestimated this in the calculation. Meaning the age of the sample was underestimated - it would be older than 950 years.
(Greater concentration → greater count rate → longer time to decay → underestimated age).