Honors Chemistry - Unit 5: Periodic Table
1/31
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
J.W. Dobereiner
Organized elements in triads, these elements share similar properties and are in groups of 3.
John Newlands
Came up with the law of octaves that stated that properties of elements repeat every 8 elements. This only worked for lighter elements.
Dmitri Mendeleev
Organized the periodic table according to increasing atomic mass and put elements with similar properties in groups. He kept some out of order to keep the elements with the same properties and predicted some elements properly.
Henry Moseley
He reorganized the elements by atomic number. Elements in increasing atomic number show a repeating pattern in physical and chemical properties.
Groups/Families
Vertical columns, elements in the same groups have similar properties. There are 18 groups.
Periods
Horizontal rows on the periodic table, there are 7 of these periods.
Valence Electrons
Electrons in the outermost shell. Elements in the same group have the same amount of valence electrons.
Family Names
There are some common names for families of elements. These are labeled on the image, make sure to know them.
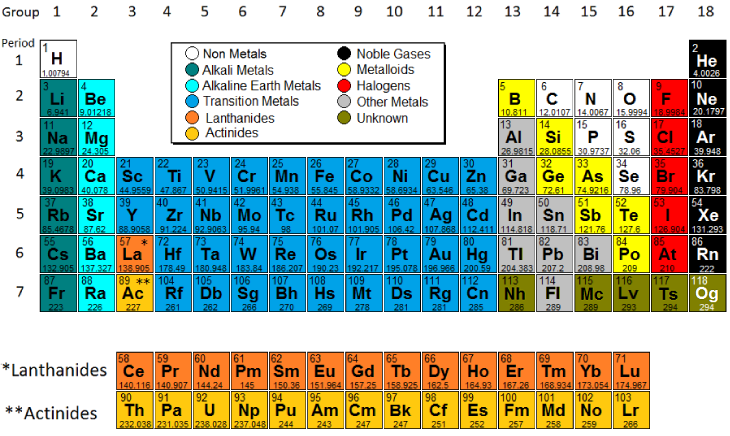
Metals
Elements that are typically shiny, good conductors of heat and electricity, and malleable. They are found on the left side and in the center of the periodic table.
Nonmetals
Elements that are generally dull, poor conductors of heat and electricity, and brittle. They are found on the right side of the periodic table.
Metalloids
Elements that have properties intermediate between metals and nonmetals, often found along the dividing line on the periodic table.
Coulombic Attraction
The force of attraction between positively charged protons in the nucleus and negatively charged electrons.
Distance
If the elements are in the same family, distance is the dominant factor. If the electrons are farther from the nucleus, the force is stronger.
Number Of Protons
If the elements are in the same family, distance is the dominant factor. The more protons, the more force of attraction.
Effective Nuclear Charge (ENC)
The force that pulls the electrons towards the nucleus, calculate the number of protons minus the core electrons.
Shielding Effect
If atoms have more than one energy level, the inner electrons will block the ENC for the outer electrons.
Bohr Model
A model of an atom, displaying its electrons and where they are.

Atomic Radius
The distance from the nucleus to the valence electrons.
Size Trend
Atoms will get larger going down the periodic table as each new groups member adds a new shell. Atoms will get smaller as you go across (left to right) because the ENC increases, causing the electrons to get pulled closer.
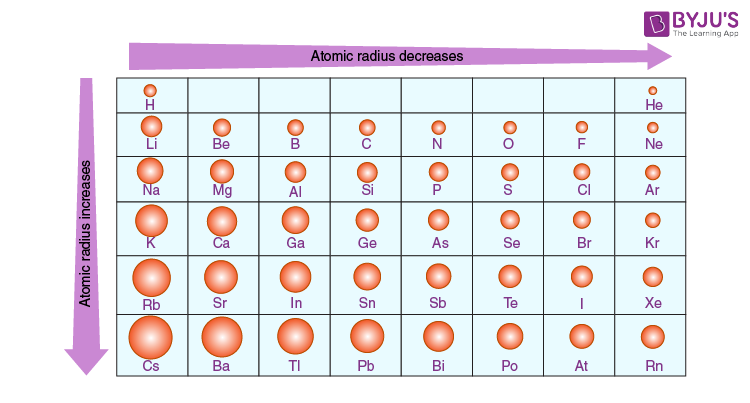
Ion Size
Ions increase in size down a group for the same reason neutral atoms do.
Ionization Energy (IE)
The energy required to remove one atom from an electron.
High IE
Elements that have high IE don’t want to lose their electrons.
Low IE
Elements that have low IE easily lose electrons.
IE Trend
IE will decrease going down a group, valence electrons get further away, making it easier to steal. IE will increase going across (left to right) as the ENC makes it harder for electrons to be stolen. IE and size have an inverse relationship.
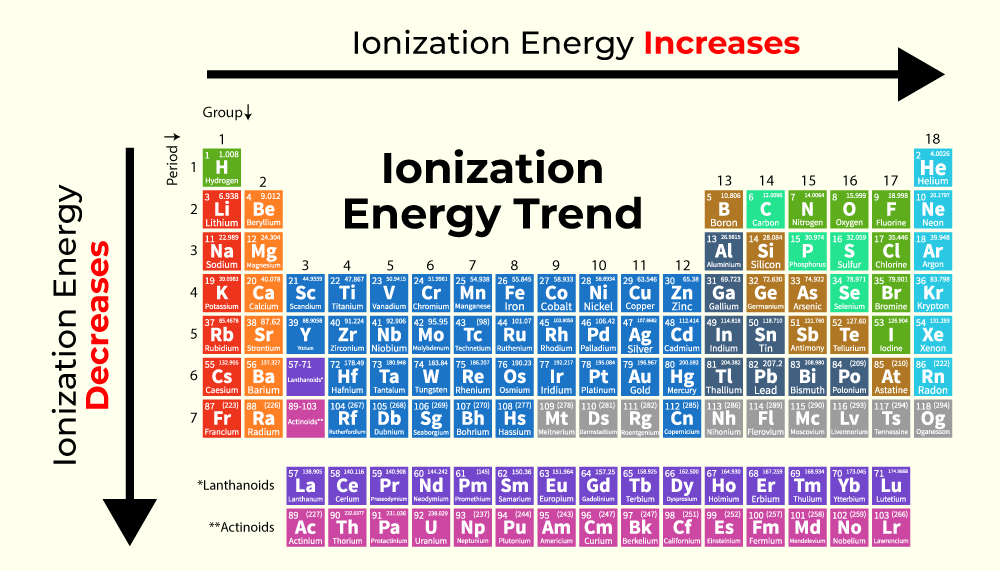
Successive IE
The amount of energy that is required to remove additional electrons beyond the first. This amount will continue to increase as it gets harder to remove electrons.
Electronegativity (EN)
The atom’s ability to attract electrons in a chemical bond.
EN Trend
The higher the EN, the greater the ability to pull electrons closer to the nucleus. The lower the EN, the lesser the ability. EN follows the exact same trend as ionization energy on the periodic table.
Metal/Nonmetal Stability
Metals lose electrons to become stable, as nonmetals share electrons or steal to become stable.
Metal/Nonmetal Reactivity
Since metals want to lose electrons, they are the most reactive, the lower the ionization energy, the more reactive the metal is. Nonmetals want to steal electrons, making them fairly nonreactive, the higher the electronegativity, the more reactive the nonmetal is.
Oxidation Number
The number that will result in the atom being stable. Basically, Calcium will need to lose 2 electrons to become stable, giving it an oxidation number of +2.
Cation
Positively charged ion.
Anion
Negatively charged ion.