Chemistry Exam
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
What ionic compound will be formed from the Zn2+ cation and the P3- anion?
A. ZnP
B. Zn2P
C. Zn3P2
D. Zn2P3
C. Zn3P2
What is the name of the ionic compound FeCl3?
A. Iron chloride
B. Iron(III) chloride
C. Iron trichloride
D. Monoiron trichloride
B. Iron(III) chloride
What is the name of the molecular compound GeBr4?
A. Germanium tetrabromide
B. Monogermanium tetrabromide
C. Tetrabromine monogermanid
A. Germanium tetrabromide
What is the formula of the molecular compound dinitrogen pentoxide?
A. N5O
B. NO5
C. N2O5
C. N2O5
5. What monatomic ion will be formed by radium (Ra), assuming the octet rule is followed?
A. Ra4+
B. Ra2+
C. Ra6-
D. Ra16-
B. Ra2+
What monatomic ion will be formed by iodine (I), assuming the octet rule is followed?
A. I17-
B. I7+
C. I-
D. I3-
C. I-
The atomic mass of hydrogen (H) is 1 u, and the atomic mass of phosphorus (P) is 31 u. What is the molecular mass of PH3?
A. 32 u
B. 33 u
C. 34 u
D. 35 u
C. 34 u
How many electrons are shared between a pair of atoms held together by a triple bond?
A. 1
B. 2
C. 3
D. 4
E. 5
F. 6
F. 6
Which of the following is a correct Lewis structure for the molecule PH3?
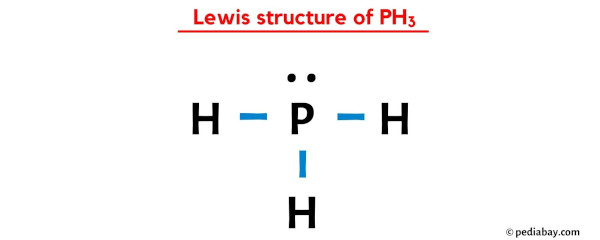
A.
The Lewis structure of methane (CH4) is shown below. According to VSEPR theory, what is the geometry at the central carbon (C) atom?
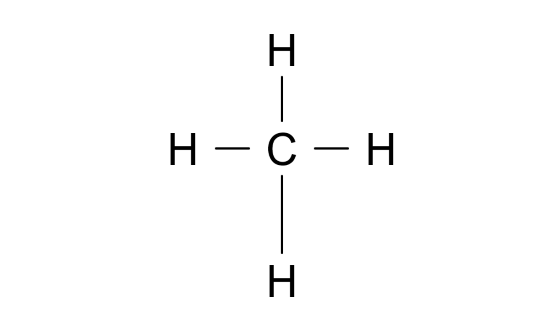
A. Square planar
B. Tetrahedral
C. Trigonal pyramidal
B. Tetrahedral
Carbon dioxide (CO2), whose Lewis structure is shown below, has a linear geometry. Each carbon-oxygen bond is strongly polar. Does a CO2 molecule have an overall zero or nonzero molecular dipole moment?
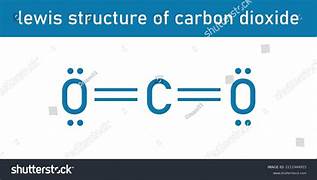
A. It has a zero molecular dipole moment.
B. It has a nonzero molecular dipole moment.
A. It has a zero molecular dipole moment.
Which of the following elements is the most electronegative?
A. Silicon (Si)
B. Phosphorus (P)
C. Sulfur (S)
D. Chlorine (Cl)
D. Chlorine (Cl)
Which of the following elements is the most electronegative?
A. Nitrogen (N)
B. Phosphorus (P)
C. Arsenic (As)
D. Antimony (Sb)
E. Bismuth (Bi)
A. Nitrogen (N)
Consider the following unbalanced chemical equation for the reaction of iron with aluminum: Al (s) + O2 (g) → Al2O3 (s). Balance this chemical equation, using the smallest possible whole-number coefficients for each reactant and product. Once the equation is balanced, what coefficient is present in front of Al (s)?
A. 1
B. 2
C. 3
D. 4
D. 4
Consider the following unbalanced chemical equation for the burning of hexane (C6H14) in oxygen: C6H14 (l) + O2 (g) → CO2 (s) + H2O (l). Balance this chemical equation, using the smallest possible whole-number coefficients for each reactant and product. Once the equation is balanced, what coefficient is present in front of O2 (g)?
A. 17
B. 19
C. 21
D. 23
B. 19
Which elemental halogen will not react with MgBr2 in a single-displacement reaction?
A. F2
B. Cl2
C. I2
D. All of the above will react with MgBr2.
C. I2
Which metal will react with Fe(NO3)2 in a single-displacement reaction?
A. Zinc (Zn)
B. Palladium (Pd)
C. Mercury (Hg)
D. All of the above
A. Zinc (Zn)
Which of the following ionic compounds will be soluble in water?
A. Na3PO4
B. Ca3(PO4)2
C. FePO4
A. Na3PO4
Which of the following ionic compounds will not be soluble in water?
A. MgBr2
B. CaBr2
C. PbBr2
C. PbBr2
How many argon atoms are in 3.20 mol argon?
A. 8.23 × 1023
B. 1.93 × 1024
C. 5.89 × 1024
B. 1.93 × 1024
Copper(II) sulfate (CuSO4) has a molar mass of 160 g/mol. What is the mass of 2.25 mol CuSO4?
A. 123 g
B. 248 g
C. 360 g
C. 360 g
Lead(II) iodide (PbI2) has a molar mass of 461 g/mol. How many moles of PbI2 are present in 98.3 g?
A. 0.13 mol
B. 0.21 mol
C. 0.38 mol
B. 0.21 mol
Elemental phosphorus (P4) and oxygen (O2) react at according to the following balanced chemical equation: P4 (g) + 5 O2 (g) → P4O10 (g). How many moles of P4 are needed to react with 4.50 mol O2?
A. 0.900 mol
B. 1.80 mol
C. 3.60 mol
D. 7.20 mol
A. 0.900 mol
Elemental carbon (C) and hydrogen (H2) react to form methane (CH4) according to the following balanced chemical equation: C (s) + 2 H2 (g) → CH4 (g). In a mixture of 1.50 mol C and 2.00 mol H2, which substance is the limiting reactant?
A. C
B. H2
C. Neither C nor H2 is the limiting reactant?
B. H2
A chemist isolates 18 g of a product from a reaction whose theoretical yield is 36 g of that product. What was the percent yield?
A. 25%
B. 50%
C. 75%
D. 100%
B. 50%