Proteins
Proteins are macromolecules composed of long chains of amino acids. Animals can synthesise some amino acids, but other essential amino acids must be ingested. Plants require a source of fixed nitrogen e.g. nitrate ions from the soil, to synthesise all the amino acids they need.
Role of proteins:
Enzymes e.g. catalase
Antibodies e.g. immunoglobin A
Hormones e.g. insulin
Transport e.g. haemoglobin
Within cell membranes e.g. channel proteins
Structural roles e.g. collagen, keratin.
Amino acids
Amino acids are composed of a carbon bonded to an amine (amino) group, a carboxyl (carboxylic acid) group, a hydrogen atom, and a variable R group. The R group is different in each amino acid.
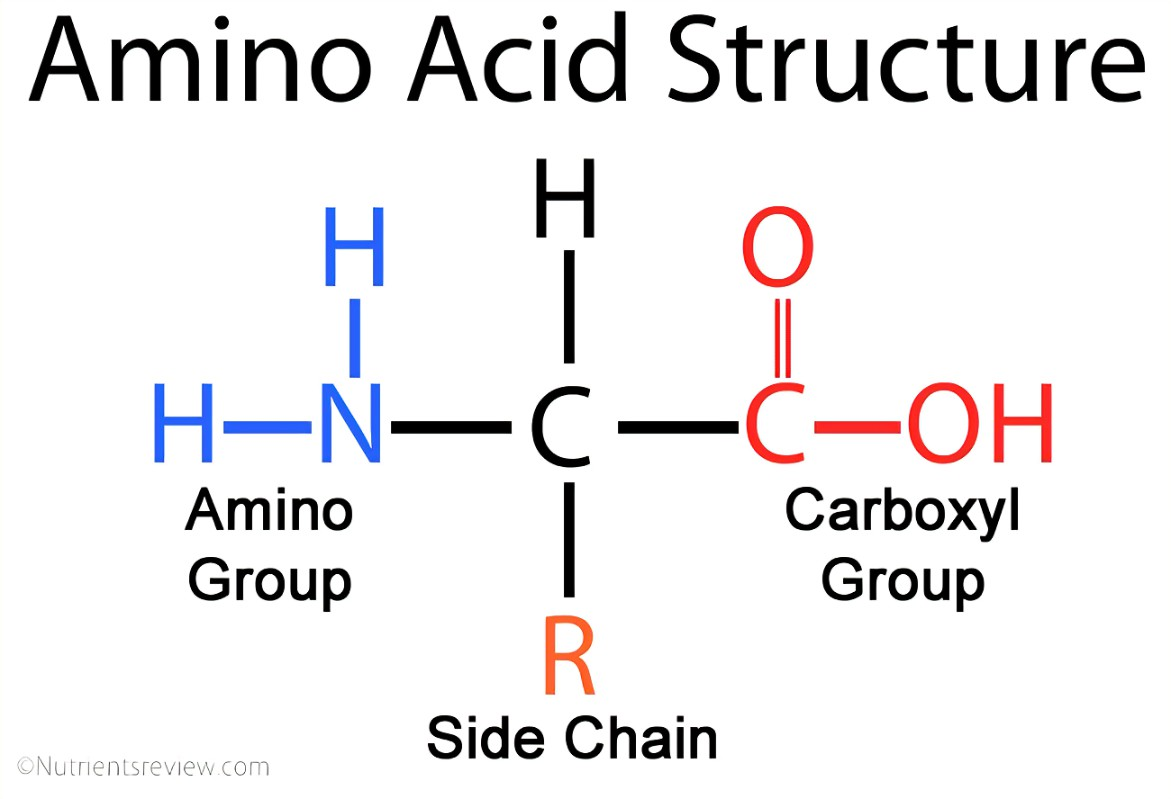
Amino acids are amphoteric as the amine group may act as a base and accept a hydrogen ion while the carboxylic group may act as an acid and donate a hydrogen ion. This allows some amino acids to act as buffers and resist a change in pH.
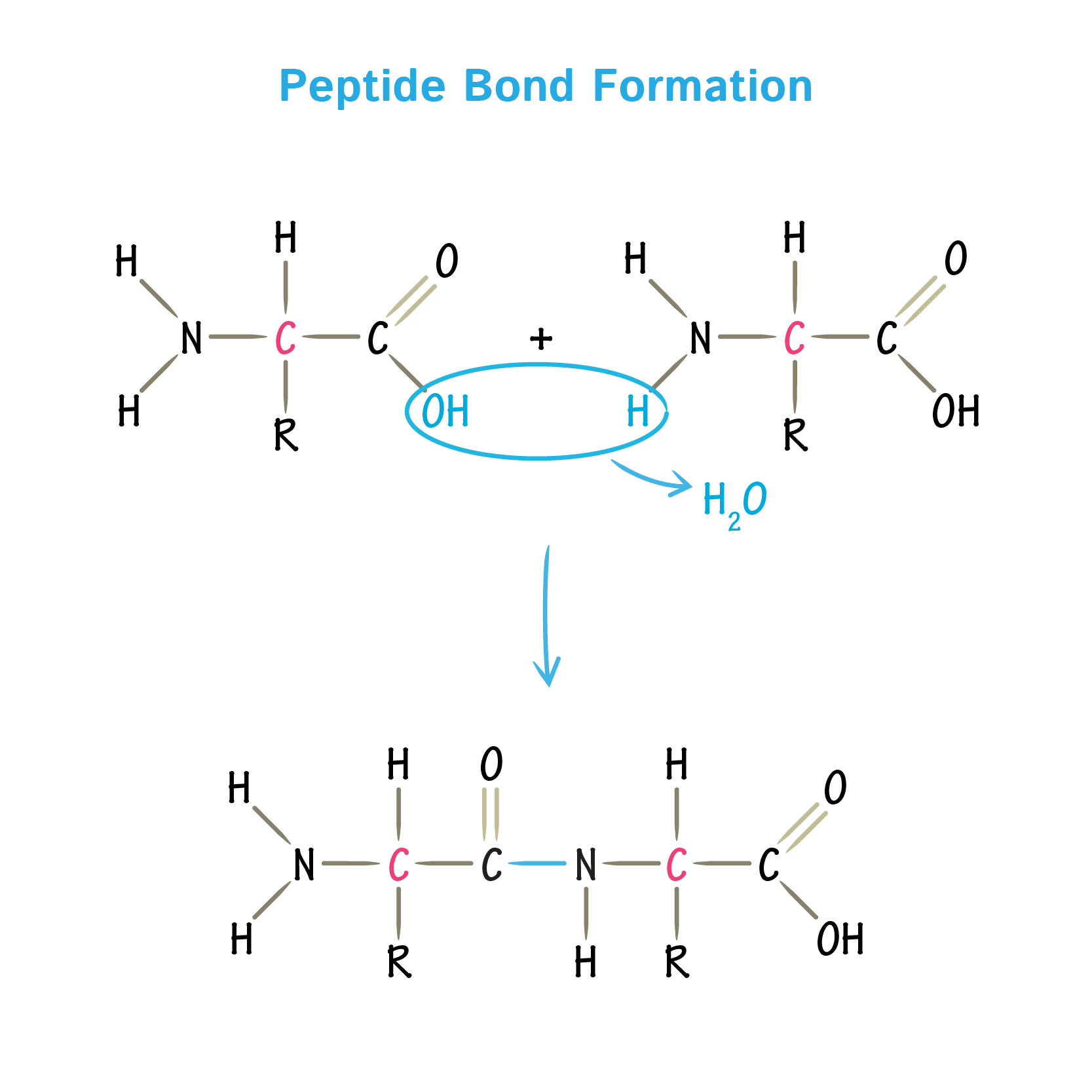
Amino acids are the monomers of proteins. They are joined together in a condensation reaction at a ribosome to form a chain of amino acids or polypeptide. Proteins may contain one or more polypeptide chains. Polypeptides are hydrolysed by protease enzymes e.g. in lysosomes and in digestion. A peptide bond forms between the amine group of one amino acid and the carboxyl group of another amino acid. This repeats many times to give a polypeptide.
Protein synthesis
Protein synthesis occurs in 2 stages. First transcription occurs in the nucleus where the gene is first copied into mRNA. The mRNA leaves the nucleus via a nuclear pore and assembles with a ribosome. The second stage is called translation and occurs at a ribosome in the cytoplasm. The ribosome synthesises the polypeptide chain by joining the amino acids together by peptide bonds, in the correct order as directed by the mRNA. A gene is a short section of DNA that codes for a polypeptide.
Protein structure
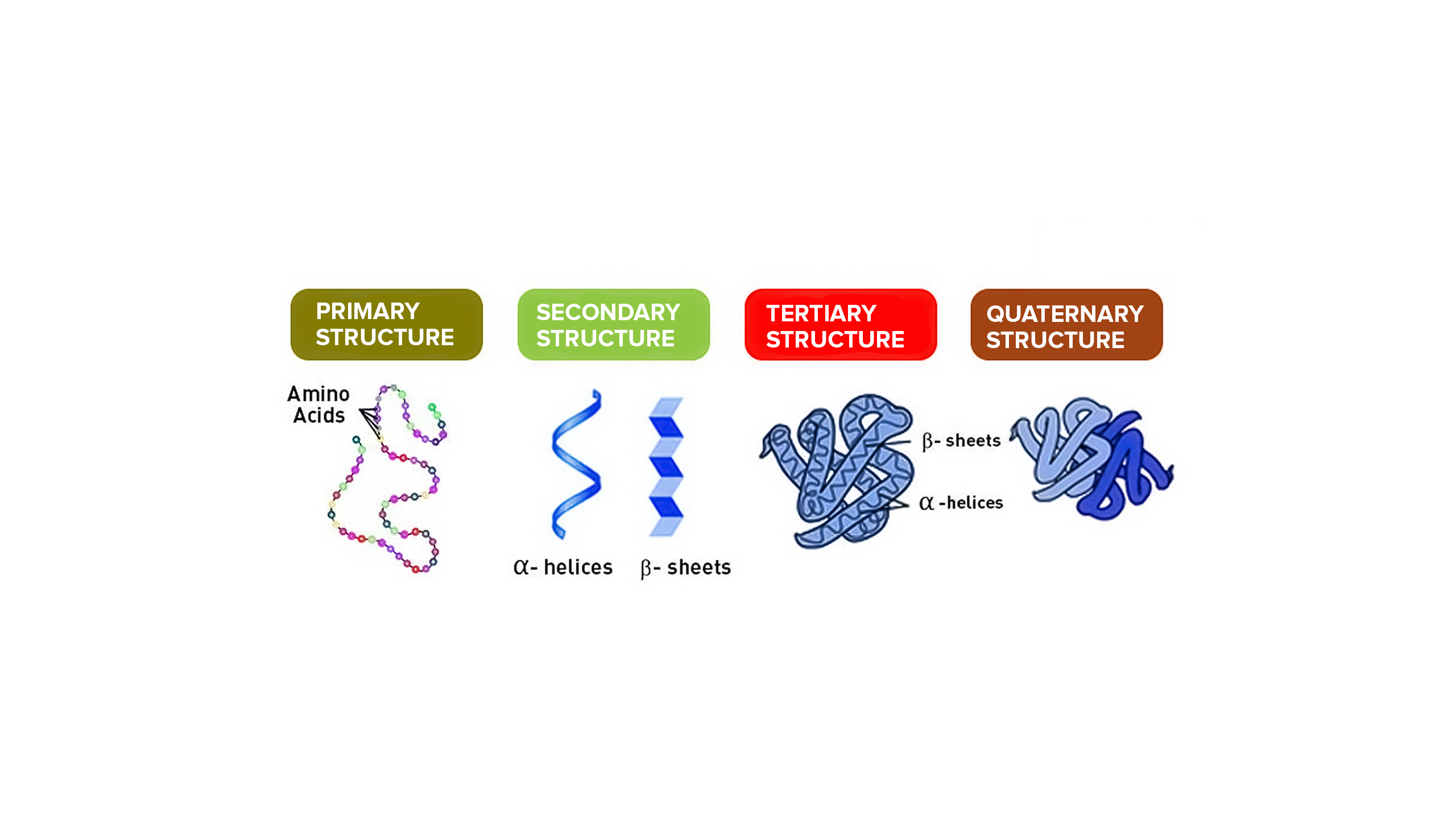
There are 4 levels of protein structure: Primary, secondary, tertiary and quaternary.
Primary structure: Order of amino acids in a polypeptide chain, which is determined by the order of bases in a gene in DNA.
Secondary structure: Regular coiling or folding of the polypeptide chain to form an α helix or ß pleated sheet. This structure is held by hydrogen bonds.
Tertiary structure: Further irregular coiling and folding of the polypeptide chain to give a specific 3D shape due to bonds between R-groups.
Quaternary structure: Linking of more than one polypeptide chain to form a protein. This occurs in the Golgi body.
There are 4 bonds that hold these structures together:
Disulfide bonds: Covalent bond between sulfur atoms in R groups of 2 cysteine amino acids. These are the strongest bonds.
Ionic bonds: Electrostatic attraction between NH₃⁺ on a basic amino acid and COO⁻ on an acidic amino acid. These are stronger than hydrogen bonds and hydrophobic interactions but weaker the disulfide bonds.
Hydrogen bonds: Weak electrostatic attraction between partial charges on electronegative oxygen atoms (in O-H and C=O) and electropositive hydrogen atoms (in NH and OH). These are stronger than hydrophobic interactions but weaker than disulfide and ionic bonds.
Hydrophobic interactions: Between non-polar R groups. These are the weakest bonds.
The primary structure is maintained by peptide bonds only. The secondary structure is held together by hydrogen bonds only. Tertiary and quaternary structure is held by disulfide, ionic, hydrogen bonds and hydrophobic interactions.
A conjugated protein is a protein that has a non-protein group attached to it (e.g. haem group on haemoglobin). This modification occurs in the Golgi body.
Fibrous proteins
Properties of fibrous proteins:
Polypeptide chains arranged in parallel with little or no tertiary folding.
Chain length may vary.
Usually insoluble in water.
Stable.
Have a structural role.
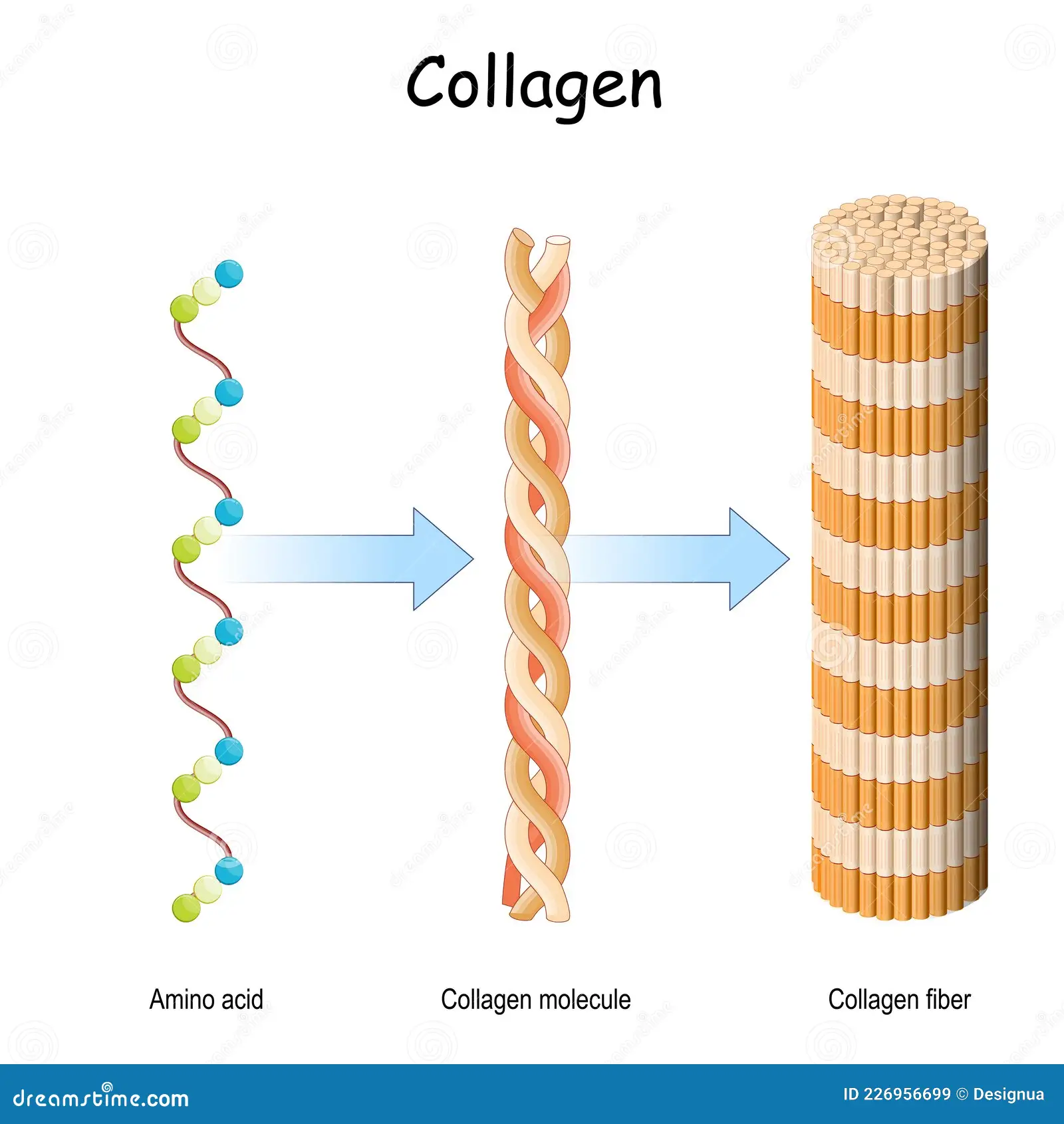
Collagen:
Collagen fibres are formed from many collagen molecules. They have a high tensile strength and provide mechanical strength. They are found in tendons, bones (reinforced with calcium phosphate to become hard), cartilage, the wall of arteries (to withstand pressure) and connective tissue.
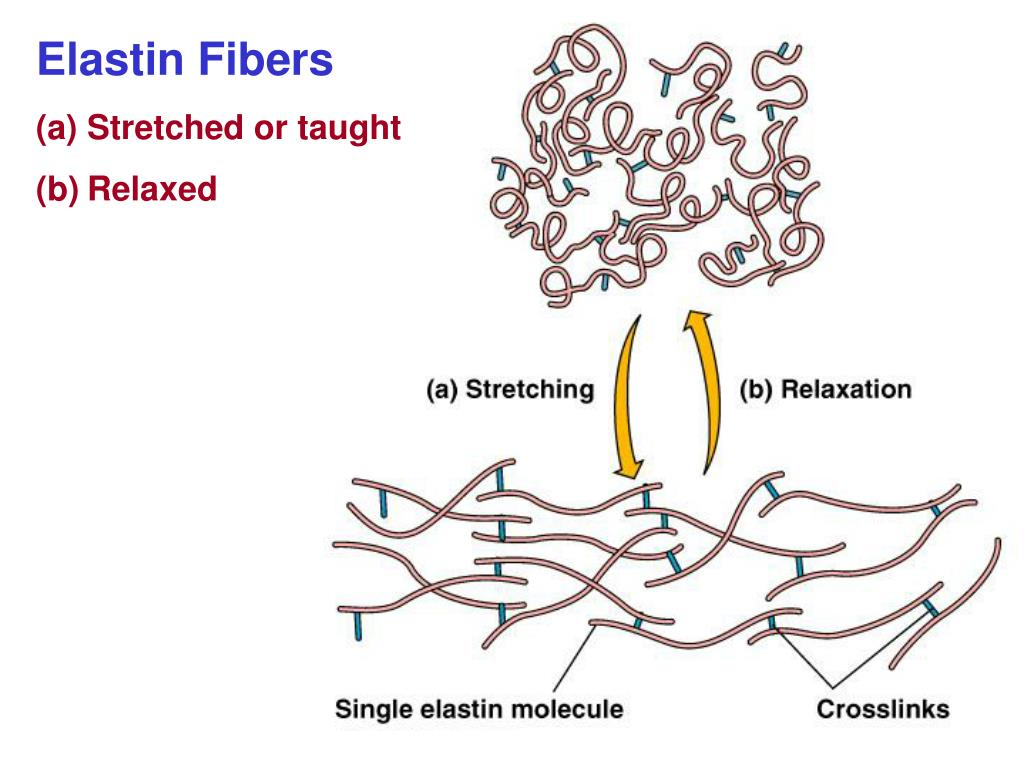
Elastin:
Elastin fibres are strong and extensible. They are found in the skin, arteries, bronchioles and alveolar walls to allow stretch and recoil.
Keratin:
Insoluble, strong keratin filaments provide mechanical protection and form a waterproof barrier. They are found in nails, hair, fur, scales, hooves, claws, and feathers.
Globular proteins
Globular proteins properties:
Polypeptide chains have a tertiary structure and fold into a specific, compact spherical shape.
Usually soluble in water.
Usually less stable (denature easily).
Often have a transport or metabolic role.
Hydrophobic (non-polar) R groups are located on the inside of the protein. They interact with each other (form hydrophobic interactions) and exclude water. Hydrophilic (polar/charged) R groups are located on the outside of the protein, where the interact (form hydrogen bonds) with water molecules. This means that globular proteins are usually soluble in proteins. However an exception to this is integral membrane proteins, which would have hydrophobic R groups on the outside where they can interact with the hydrophobic phospholipid tails.
Globular proteins are less stable than fibrous proteins because their tertiary structure is held by hydrogen and ionic bonds which can be broken by high temperature and extremes of pH so the protein denatures.
Haemoglobin:
Haemoglobin is a globular protein which has a quaternary structure as it is composed of 2 α polypeptide chains and 2 ß polypeptide chains. It is also a conjugated protein as each polypeptide chain has a covalently attached haem prosthetic group containing Fe²⁺. The role of haemoglobin is to transport oxygen from the lungs to the tissues. The oxygen binds to the iron in each haem group meaning one molecule of haemoglobin can bind 4 molecules of oxygen.
Antibodies:
Antibodies are globular proteins with quaternary structure as they contain 2 light chains and 2 heavy chains held together by disulfide bonds. They are also conjugated proteins as a carbohydrate is added to it.