Module 2 - the Molecules of the Cell
1/28
Earn XP
Description and Tags
included in exam 1
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
the 4 major families of organic molecules in the cell
sugars (form polysaccharides, glycogen, and starch)
fatty acids (form fats and membrane lipids)
amino acids (form proteins)
nucleotides (form nucleic acids)
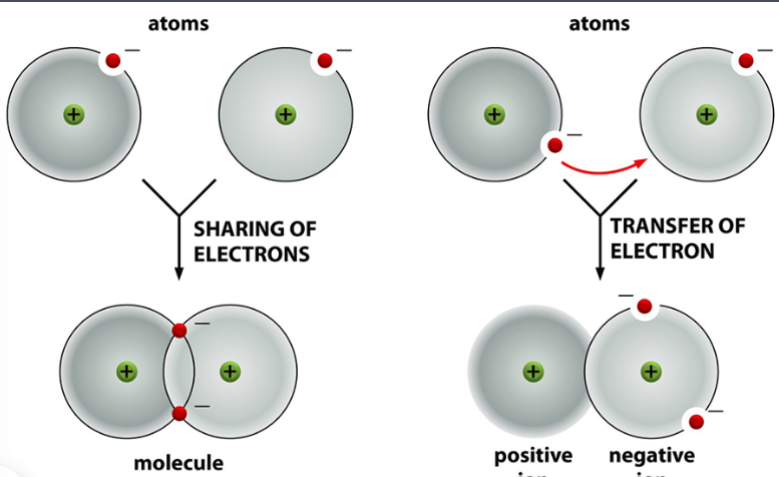
difference between covalent and ionic bonds
covalent bonds are formed through sharing of electrons
ionic bonds are formed through transfer of electrons
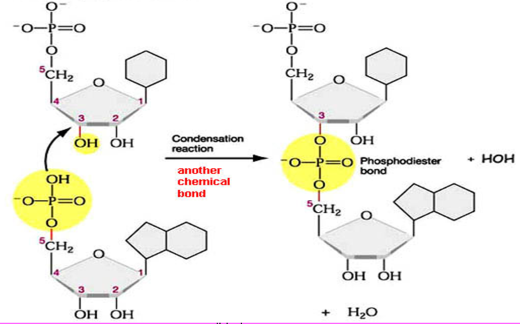
formation of macromolecules by condensation reactions
subunits are added to one end of a growing chain via dehydration synthesis (aka condensation; forms a water)
subunits are linked by covalent bonds
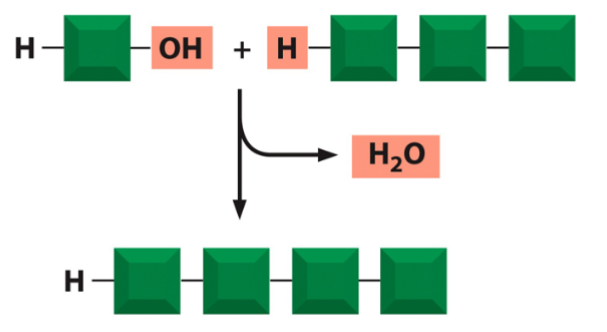
degradation of macromolecules
subunits are removed from one end of a polymer by hydrolysis (breaks covalent bonds)
the opposite of dehydration synthesis
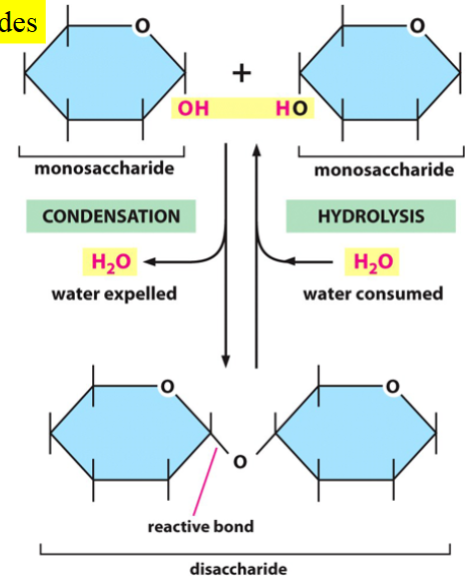
formation of disaccharides
condensation of two monosaccharides produces one disaccharide
for the forward reaction, 1 water is expelled (OH from one monosaccharide, H from the other)
for the reverse reaction, 1 water is consumed
3 types of polysaccharides
glycogen (found in animals)
starch (found in plants)
cellulose (found in plants)
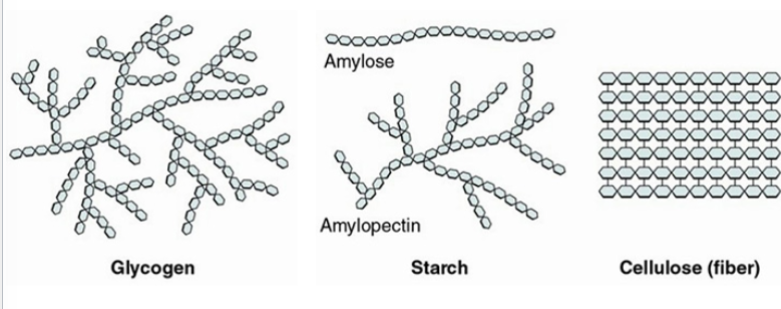
glycogen
a polysaccharide used for energy storage in animals
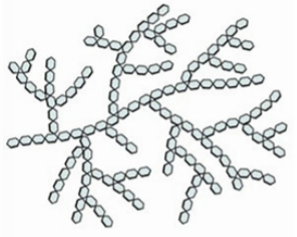
starch
a polysaccharide used for energy storage in plants
many different types, e.g. amylose, amylopectin
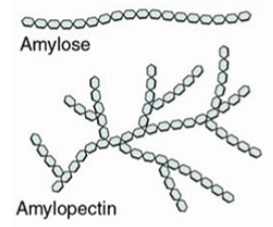
cellulose
a polysaccharide used for structure in plants
fiber
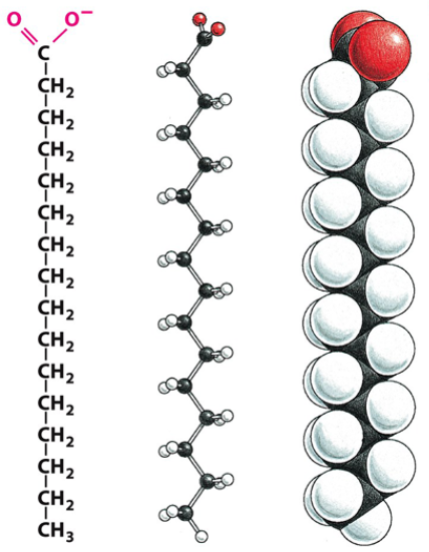
fatty acids
one of the 4 major organic molecules
form fats and lipids
consist of a carboxylic acid head (hydrophilic, indicated with red in the picture) and a hydrocarbon tail (hydrophobic, indicated with black and white in the picture)
can be saturated or unsaturated
stored as energy reserves (fats and oils) through ester linkage to glycerol to form triacylglycerols
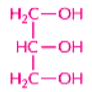
glycerol
a trihydroxy alcohol molecule involved in the formation of triacylglycerols (for the organization of fatty acids) and phospholipids
important: has 3 -OH groups, which can link via ester linkages to fatty acids or to a phosphoric acid (also through condensation reaction)
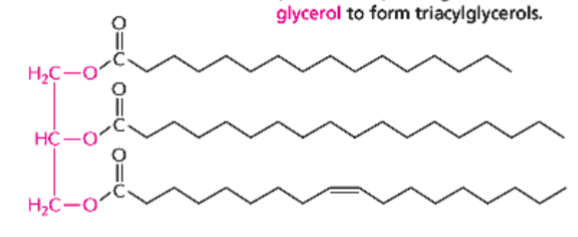
triacylglycerols
three fatty acids attached to a glycerol (indicated in red) through ester linkages
serve as energy reserves (fats and oils)
can be all saturated, all unsaturated, or a mix of the two
note: glycerol usually has OHs instead of just Os; the Hs are removed during ester linkage to form H2Os with the 2 Os removed from each fatty acid
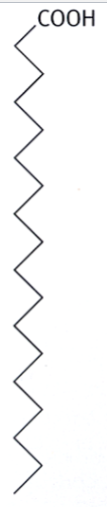
saturated fatty acids
fatty acids in which the hydrocarbon chain holds as many hydrogens as it possibly can
form a straight chain (depicted as zigzag)
tend to form solid aggregates and deposits inside blood vessels
(think: straight dry spaghetti strands, able to fit together nicely)
unsaturated fatty acids
fatty acids that contain at least one double bond; the hydrocarbon chain is not holding its maximum amount of hydrogens
can be cis or trans
(extra info: cis can be converted into trans, e.g. with the elaidinisation of oleic acid into elaidic acid)
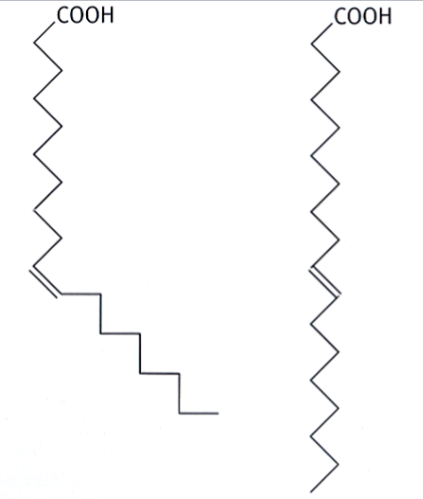
cis unsaturated fatty acids
unsaturated fatty acids in which the double bond is cis (both larger groups are on the same side of the double bond)
do not form aggregates because the double bond forms a kink/bend
(think: bent pasta shapes are difficult to fit together nicely like straight spaghetti → harder to clump together)
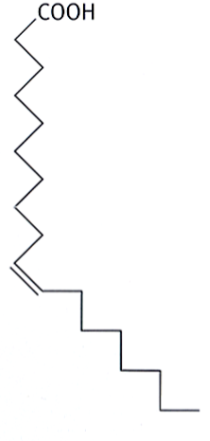
trans unsaturated fatty acids
unsaturated fatty acids in which the double bond is trans (the larger groups are on different sides of the double bond)
behave similarly to saturated fatty acids; tend to aggregate and form solid deposits (because the trans double bond makes the hydrocarbon chain linear/zigzagged)
major contributors to coronary heart disease (atherosclerosis of coronary blood vessels)
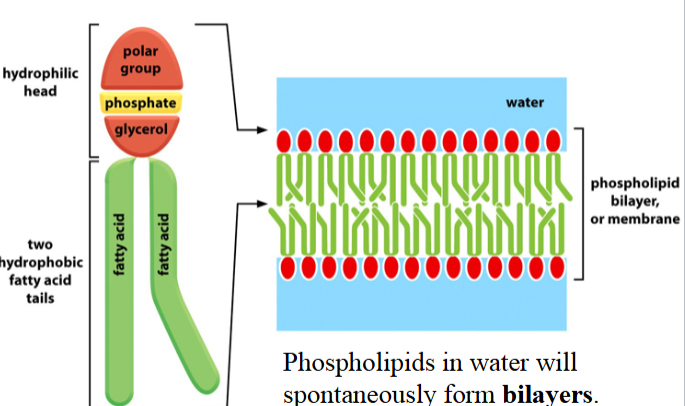
phospholipids
formed with glycerol and fatty acids
two -OH groups in glycerol are linked to fatty acids (like in a triacylglycerol)
the third -OH group in glycerol is linked to a phosphoric acid (different from a triacylglycerol)
the phosphate is linked to a small hydrophilic group (e.g. choline, in phosphatidylcholine)
in biological membranes, typically contain one saturated fatty acid and one cis unsaturated fatty acid
in water, will spontaneously form bilayers
why do phospholipids in biological membranes contain one saturated fatty acid and one cis unsaturated fatty acid?
for a balance between the effects of both
effect of saturated FAs: would make the membrane harder and less fluid
effect of cis unsaturated fatty acids: would reduce membrane stability
having one of each yields stability without being too rigid and fluidity without being too weak
nucleic acid
formed from nucleotides (one of the 4 major organic molecules)
e.g. DNA, RNA
formed through phosphodiester bonds between nucleotides (highlighted in yellow)
results in a dinucleotide (or polynucleotide, if repeated) with 5’ to 3’ polarity
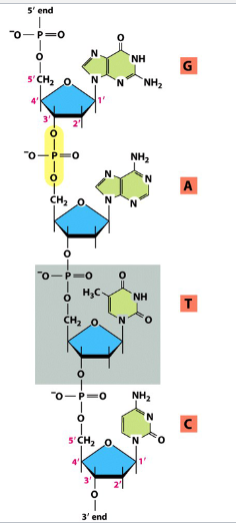
phosphodiester bond
the bond between 2 nucleotides in a nucleic acid
a phosphate group is bonded to the 3’ C of one nucleotide and to the 5’ C of the next two 2 ester linkages
C-3’ to C-5’ bond
results in a dinucleotide with 5’ to 3’ polarity
a phosphodiester has a central P instead of O, and has ORs on both sides
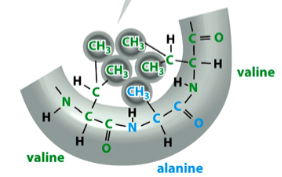
intramolecular non-covalent interactions
electrostatic interactions, hydrogen bonds, or van der Waals forces
weak, but collectively strong enough to define the folded structure of a protein
stabilize the 3D conformations that most proteins and many RNA molecules fold into
mediate interactions of proteins and nucleic acids between each other and with other molecules of compatible groups
can be disrupted by denaturants or heat (result in unstructured polymer chains)
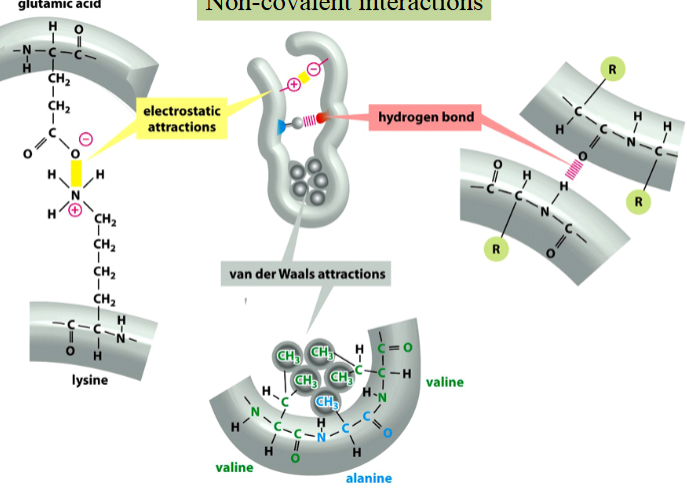
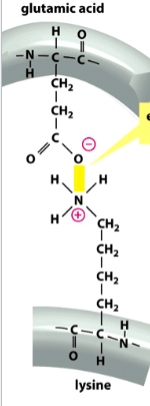
electrostatic interactions
a type of non-covalent interaction
attractions between ionized (charged) groups
depicted in yellow
hydrogen bond
a type of non-covalent interaction
the electromagnetic attractive interaction of a H atom (electron-poor) and an electronegative atom (e.g. N, O, or F) that is present in another molecule or chemical group
depicted in red/pink
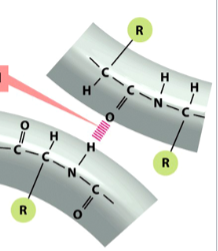
van der Waals forces
a type of non-covalent interaction
weak interactions between adjacent electrically neutral molecules caused by the attraction of electron-rich regions of one chemical group (e.g. the C in methyl) and electron-poor regions of another (e.g. the Hs in methyl)
depicted in green
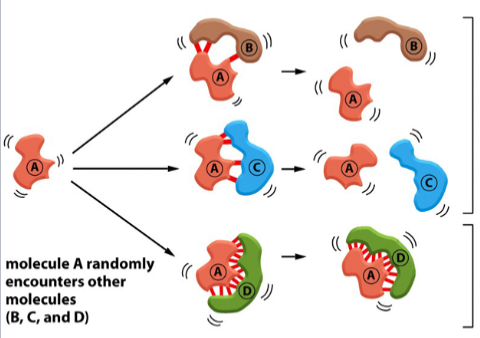
compatible groups interacting non-covalently
non-covalent bonds will be strongest when the two molecules are compatible
non-compatible molecules might bond, but only at a few points; thermal motion rapidly breaks them apart
(note: “strong” covalent bonds do not mean the bonds themselves are stronger, there are just more bonds → more collective strength)
in the picture: A and D are compatible. A is not compatible with B or C. Red lines represent non-covalent bonds
cyclic nucleoside monophosphates
monophosphates that form a phosphodiester bond between C3 and C5 of themselves
e.g. cAMP
can
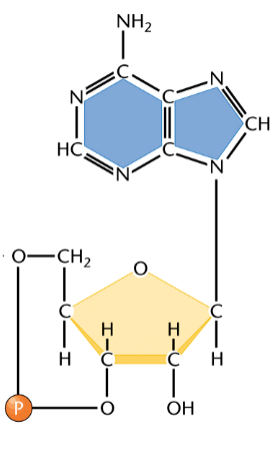
cAMP
cyclic adenosine monophosphate
a cyclic nucleoside monophosphate
used by cells as a messenger in both intracellular and intercellular signaling
can bind to a specific binding site on a protein, e.g. epinephrine, to activate the protein
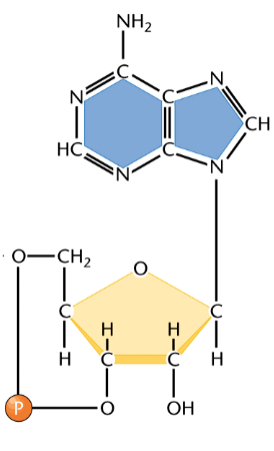
cAMP and epinephrine
epinephrine releases glucose from glycogen stores
in one step, cAMP binds to a protein and activates it
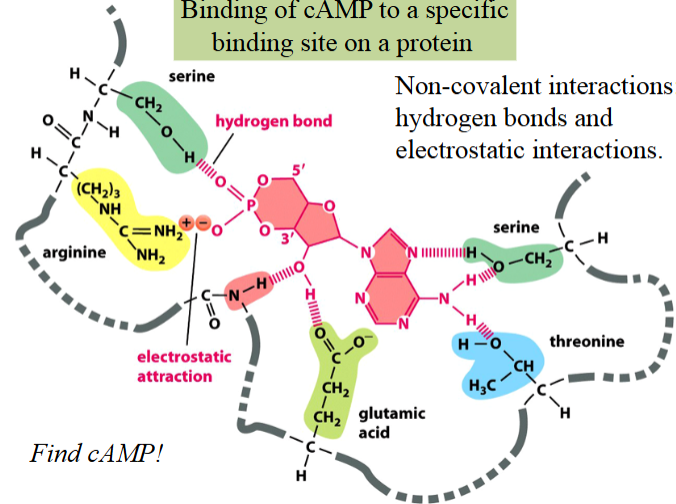
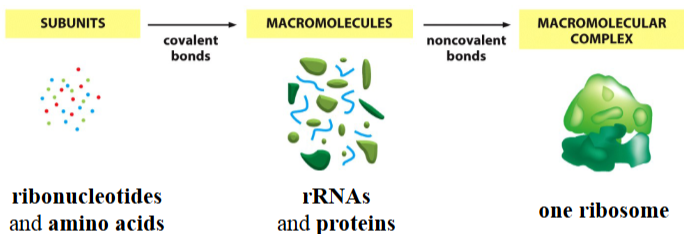
macromolecular complexes
amalgamation of different macromolecules to form one larger unit
e.g. one ribosome, made from many rRNAs and proteins, which are made from many ribonucleotides and amino acids
(extra info: 1 ribosome has 4 different rRNA species and 79 ribosomal proteins, held together by non-covalent interactions)