CHEM 2443 Key Terms & Definitions for Exam 2 Review
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
Dehydrohalogenation
Use strong base to deprotonate to make alkene (K+ takes Br, OH takes H)
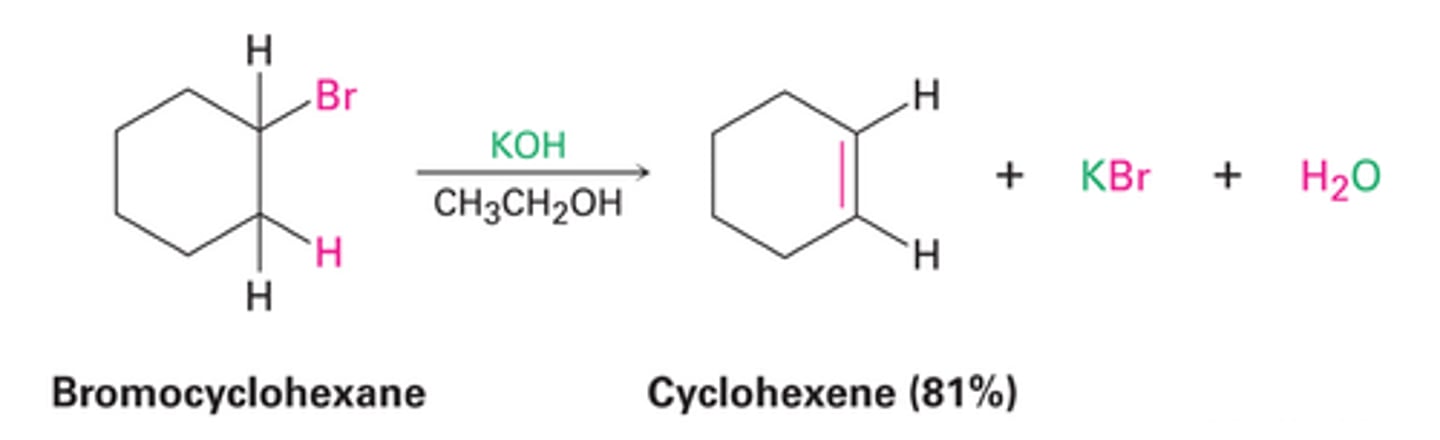
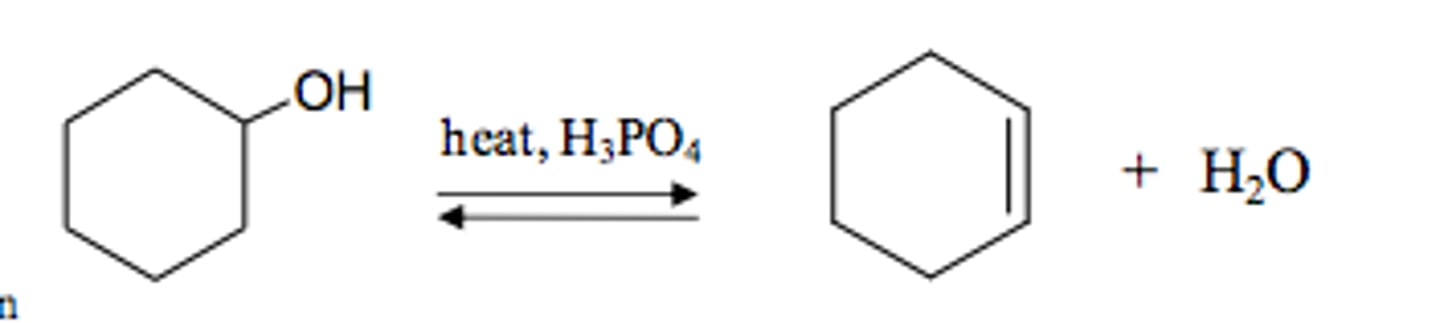
Dehydration
Use strong acid to make alkene (strong acid protonates OH, water takes H+ from nearby C to form C=C)
Reduction (which mechanism)
Gaining electron density for carbon (hydrogenation)
Oxidation (which mechanisms 5)
Losing electron density for carbon
(halogenation, halohydrination, epoxidation, dihydroxylation with OsO4, ozonolysis)
(anti or syn/trans or cis/mark or not)
give species needed/catalysts
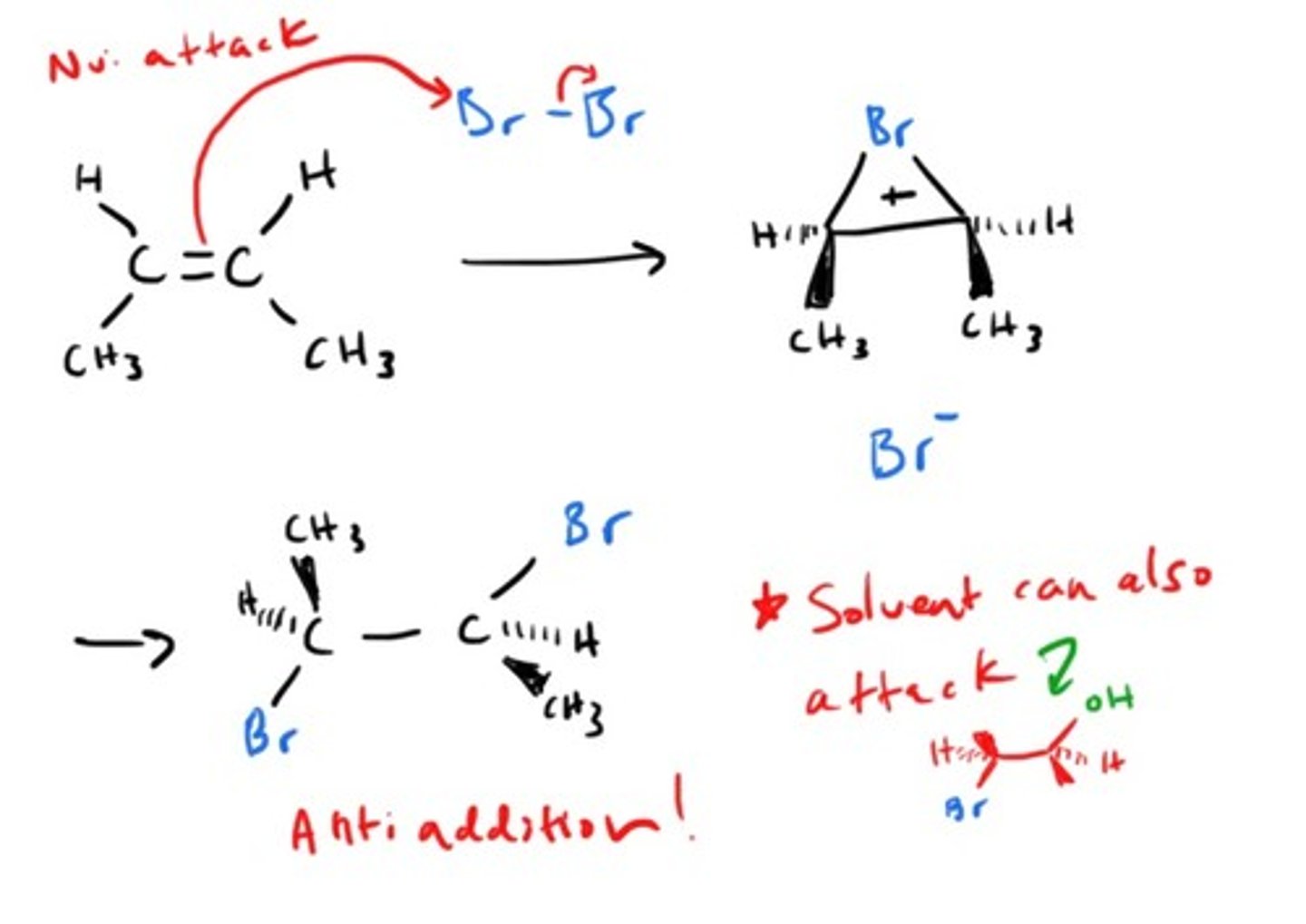
Halogenation
Anti/trans/mark - bromonium
Alkene + X2 (halogen) --> alkanes with halogen
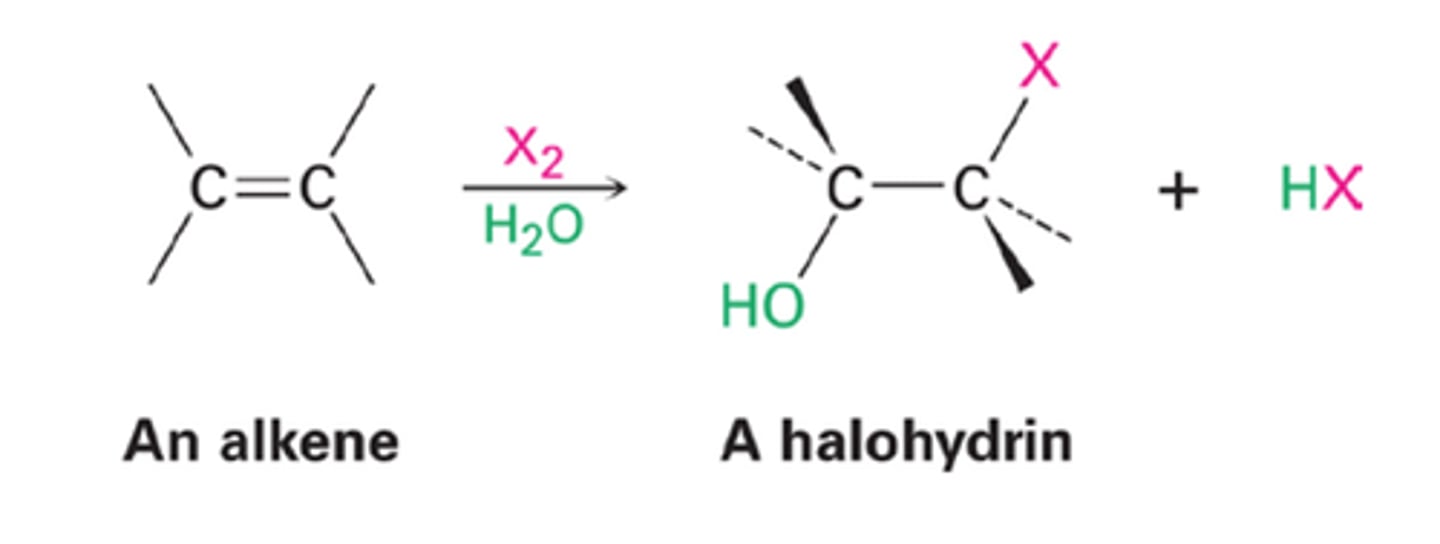
Halohydrination
Anti/trans/mark - bromonium
Alkene + X2 + H2O (DMSO) --> alkane alcohol
Halogen Nu in first attack, water Nu in second attack; extra deprotonation step)
Acid catalyzed hydration (a/chiral alkene makes...)
Anti/trans/mark
Alkene + H2O --H3O+--> alkane alcohol
Chiral alkene = chiral products; achiral alkene = racemic)
Carbocation rearrangement
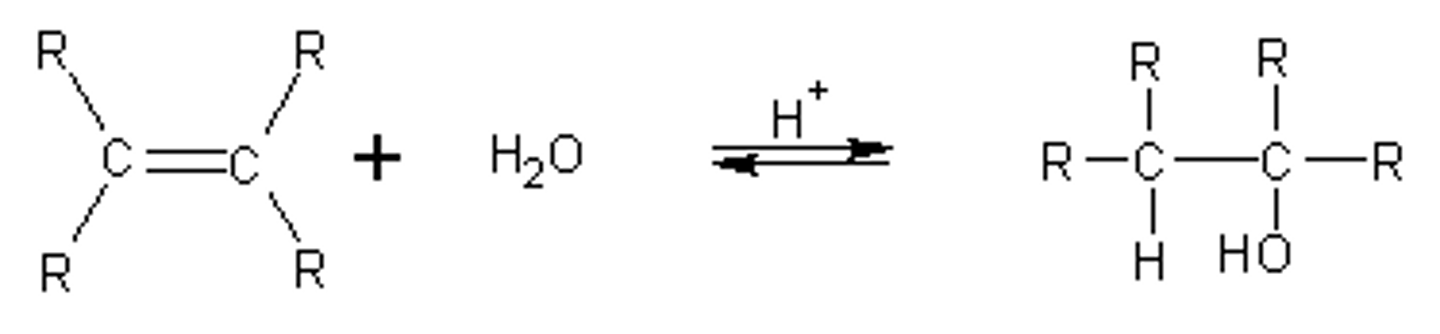
Oxymercuration-demurcuration hydration
Anti/trans/mark
Alkene + HgOAC +H2O -->NaBH4--> alkane alcohol
step 1: similar to bromonium formation
step 2: OH addition follows mark and deprotonation
step 3: NaBH4 swaps HgOAC for H
No carbocations rearrangement
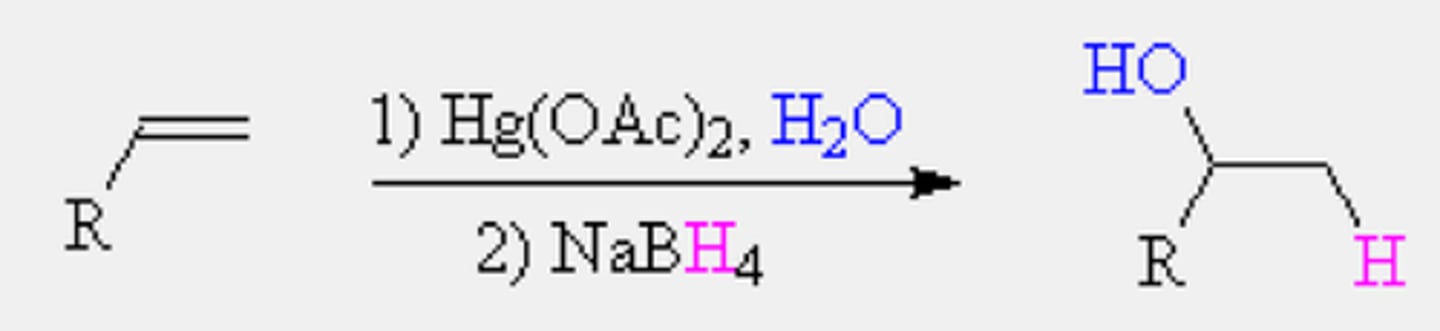
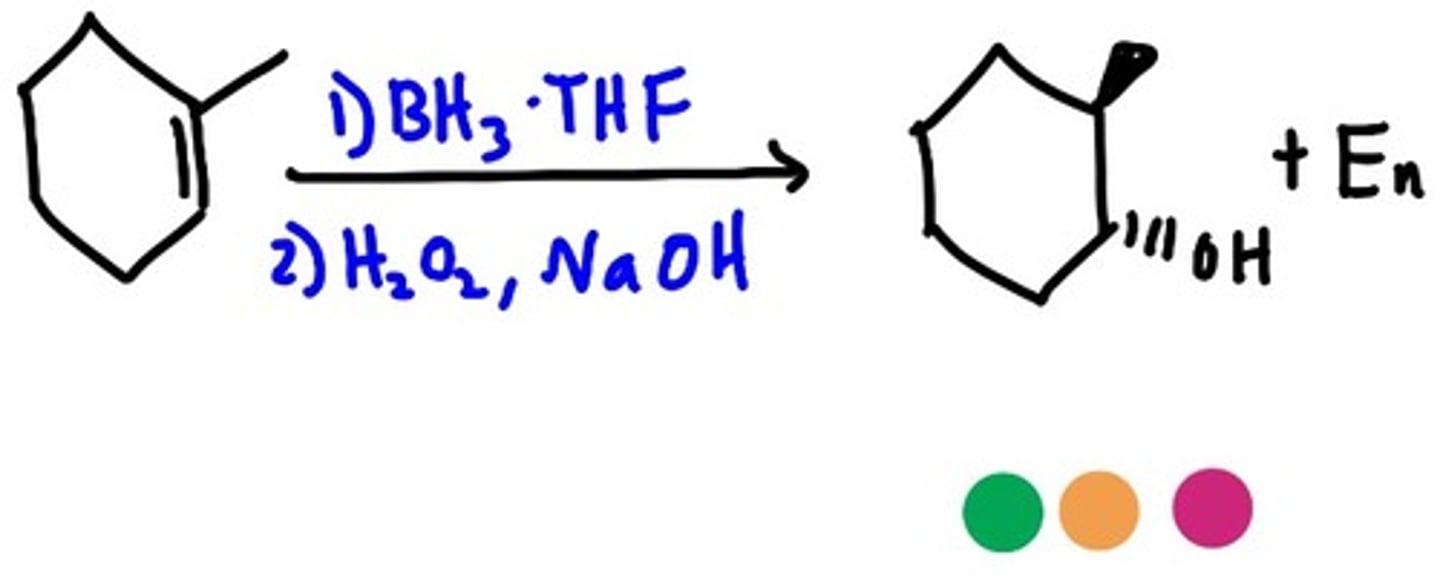
Hydroboration
Syn/cis/no mark (SN1 - one step)
Alkene + BH3 -+ THF -> oraganoborane --> H2O2 +NaOH +H2O --> alkane alcohol
(steric crowding relevant because no carbocation forms so BH2 adds to the less substituted C)
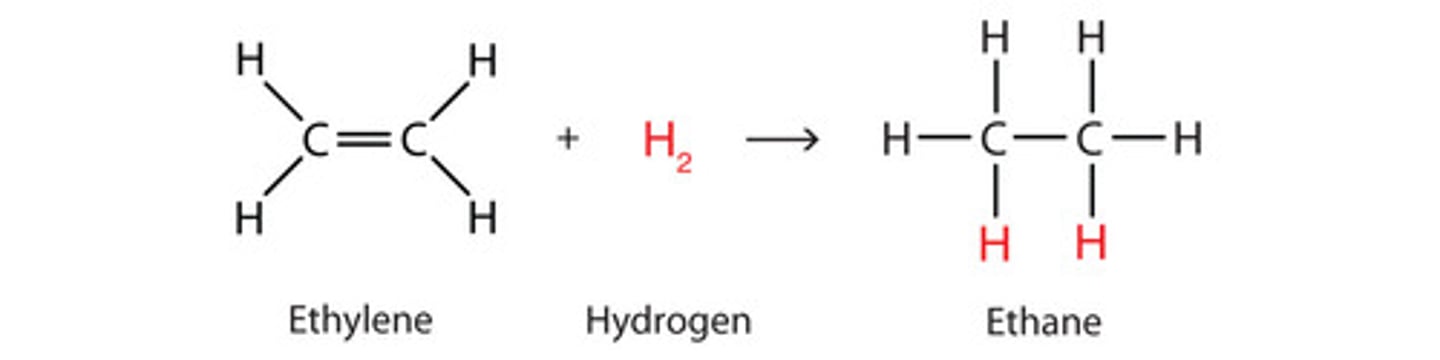
Hydrogenation
Syn/cis/mark does not apply)
Alkene + H2 --> Pd/C (metal catalyst) --> alkane
Epoxidation using peroxyacids
Anti/trans/mark
Alkene + mCPBA --> (CH2Cl) --> epoxide
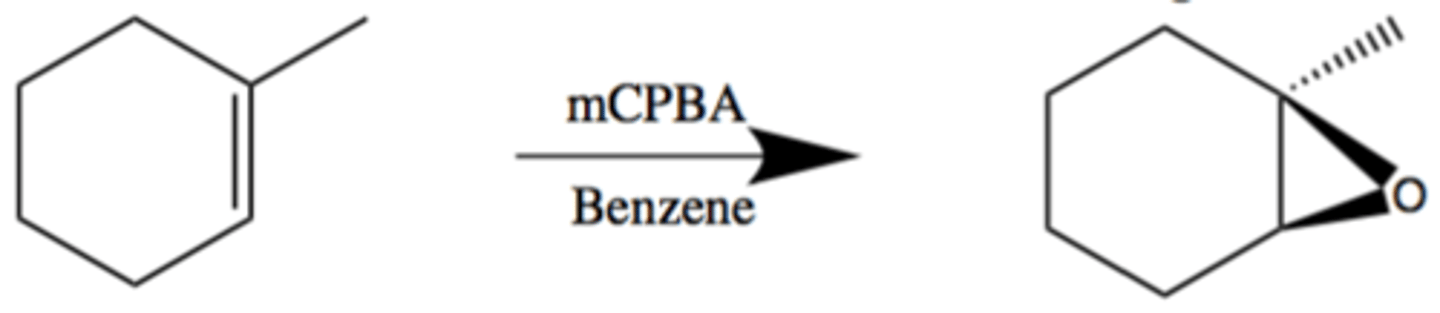
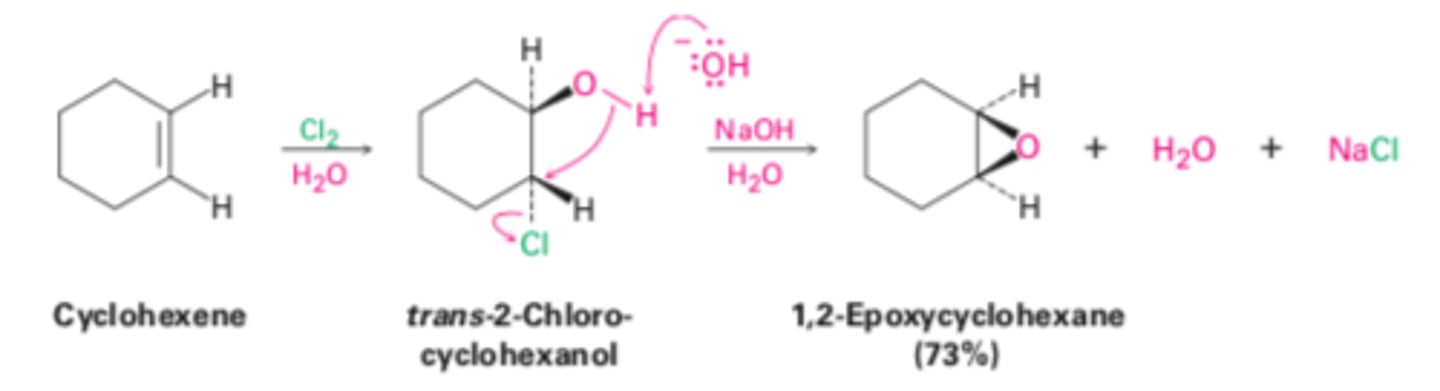
Epoxidation using halohydrins
Anti/trans/mark
Alkene + Cl2 (halogen) + H2O --> alkane
Alkane int + NaOH --> alkane + H2O + NaCl
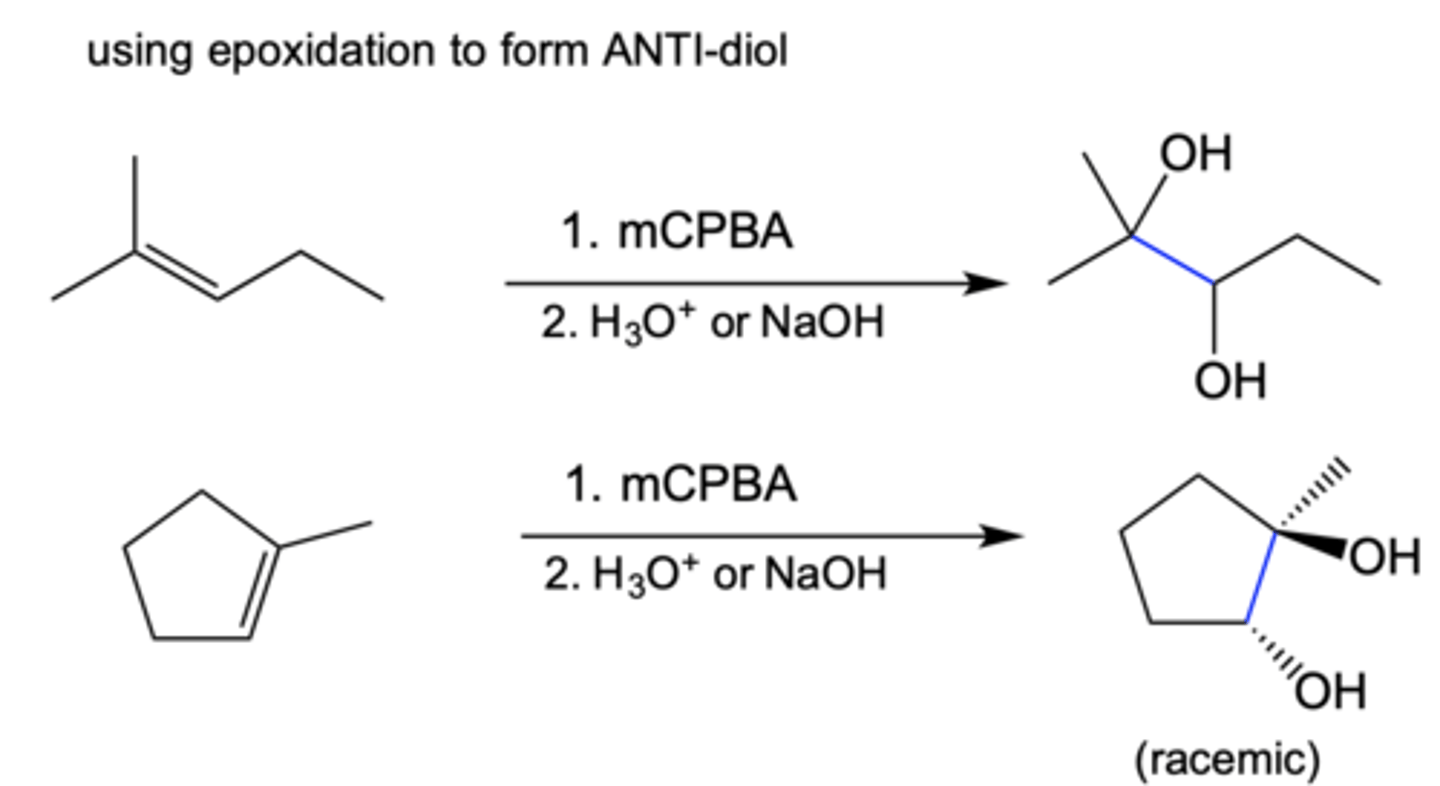
Epoxidation to make 1,2-diols (2 OHs)
Anti/trans/mark
Alkene --> epoxide
Epoxide + H3O+ --> int
H2O (Nu) then deprotonate --> alkane diol
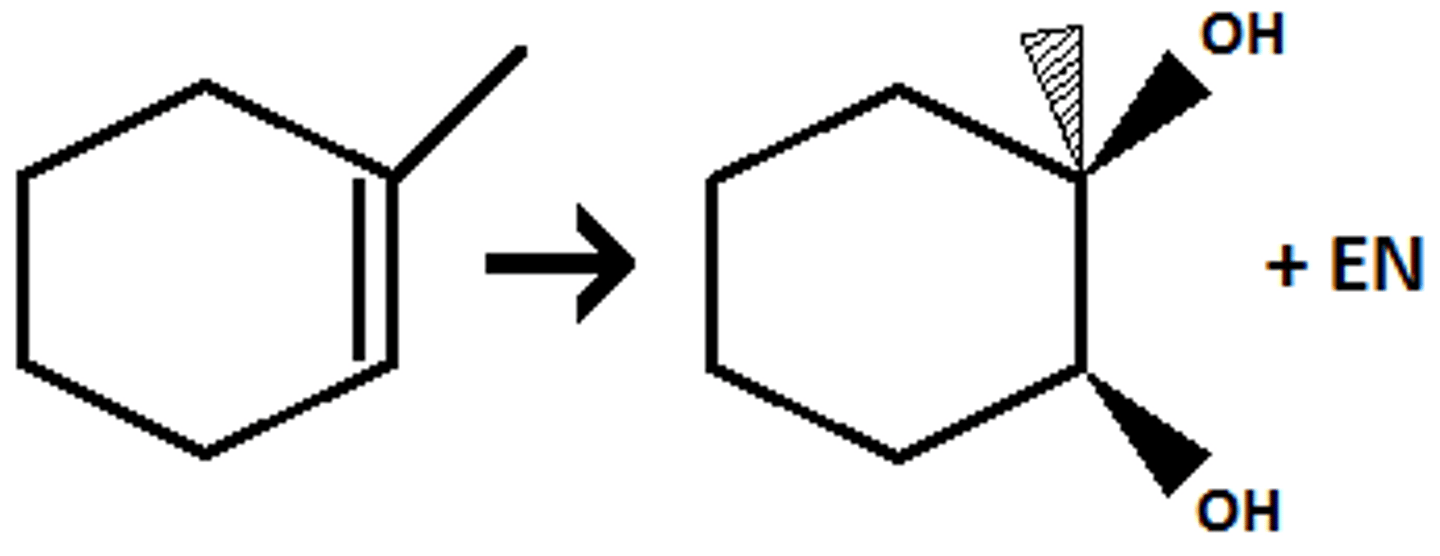
Dihydroxylation (no epoxidation)
Syn/cis
Alkene + OsO4 --> int. --> NaHSO3 + H2O --> alkane 1,2-diol
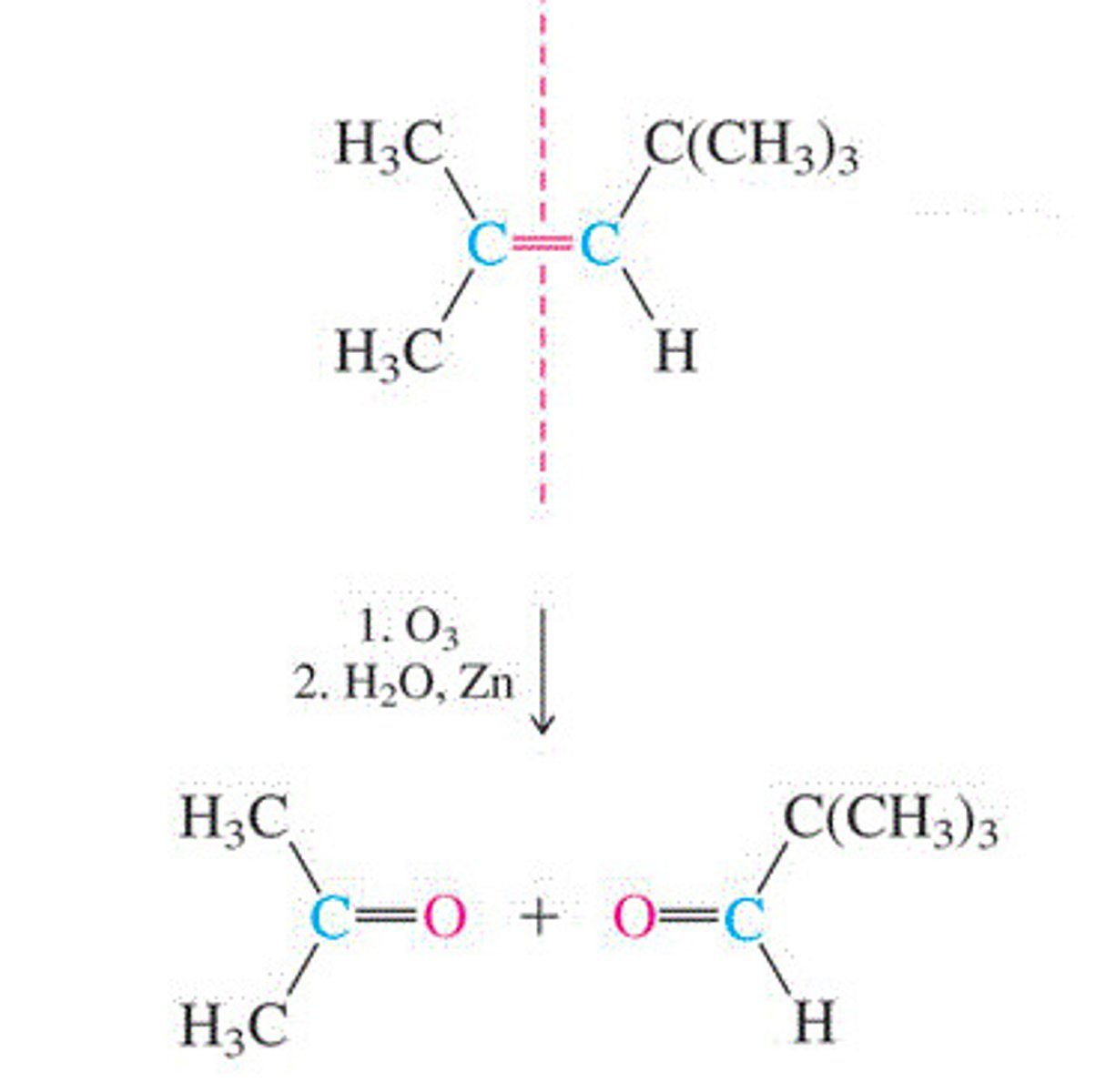
Ozonlysis
Alkene + O3 --> ozonide + H2O + Zn --> 2 alkenes
-tetrasubstituted alkenes = 2 ketons (C=O)
-disubstituted alkenes = 2 aldehydes (HC=O)
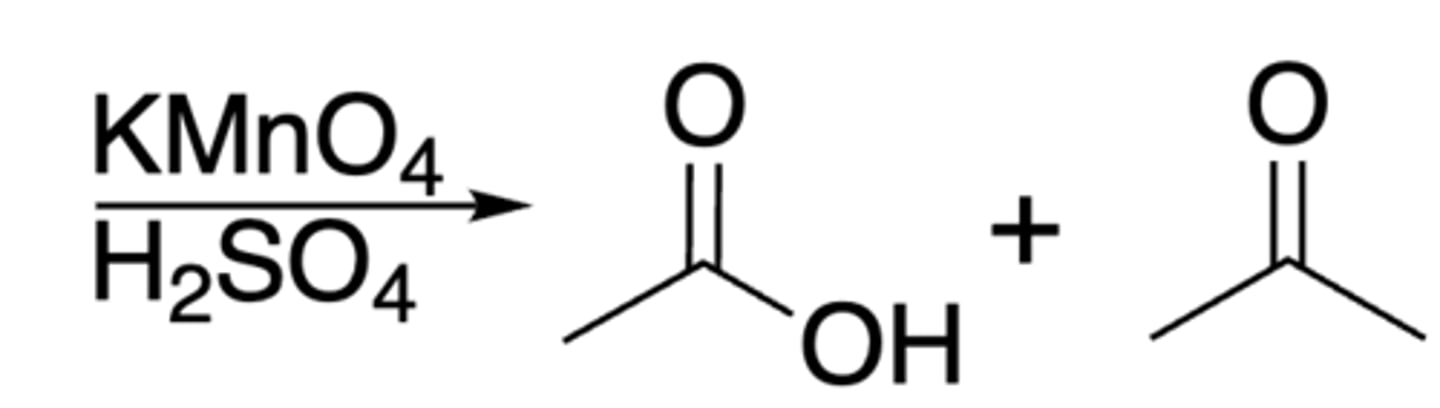
Oxidation of alkene via KMnO4
Alkene + KMnO4 + OH- + heat --> ketone + carboxylate
- at least one C on the alkene must have H attactched
- monosubstitued side turns into carboxylic acid
- disubstituted side turns into ketone
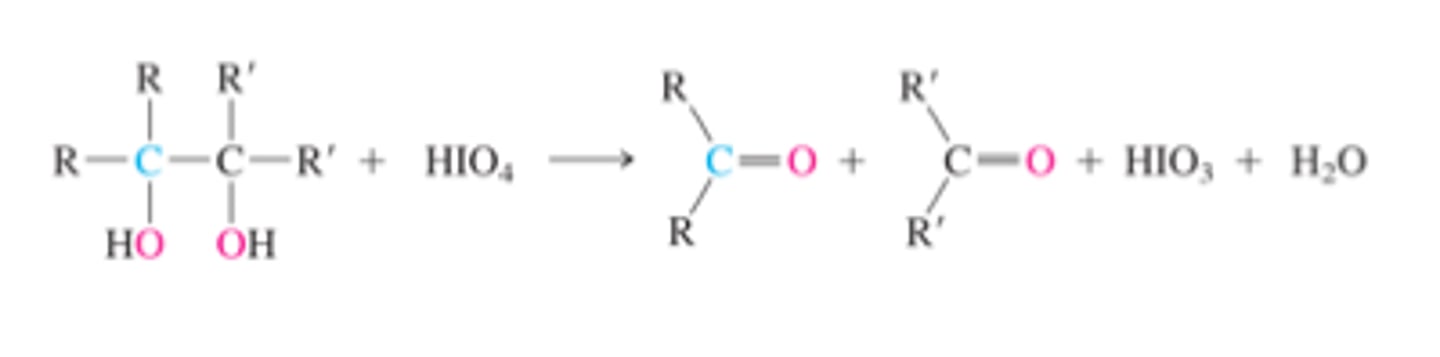
Oxidation of alkene vis 1,2-diols with HIO4
1,2-diol + HIO4 --> int. --> carbonyl
(H removed to form C=O)
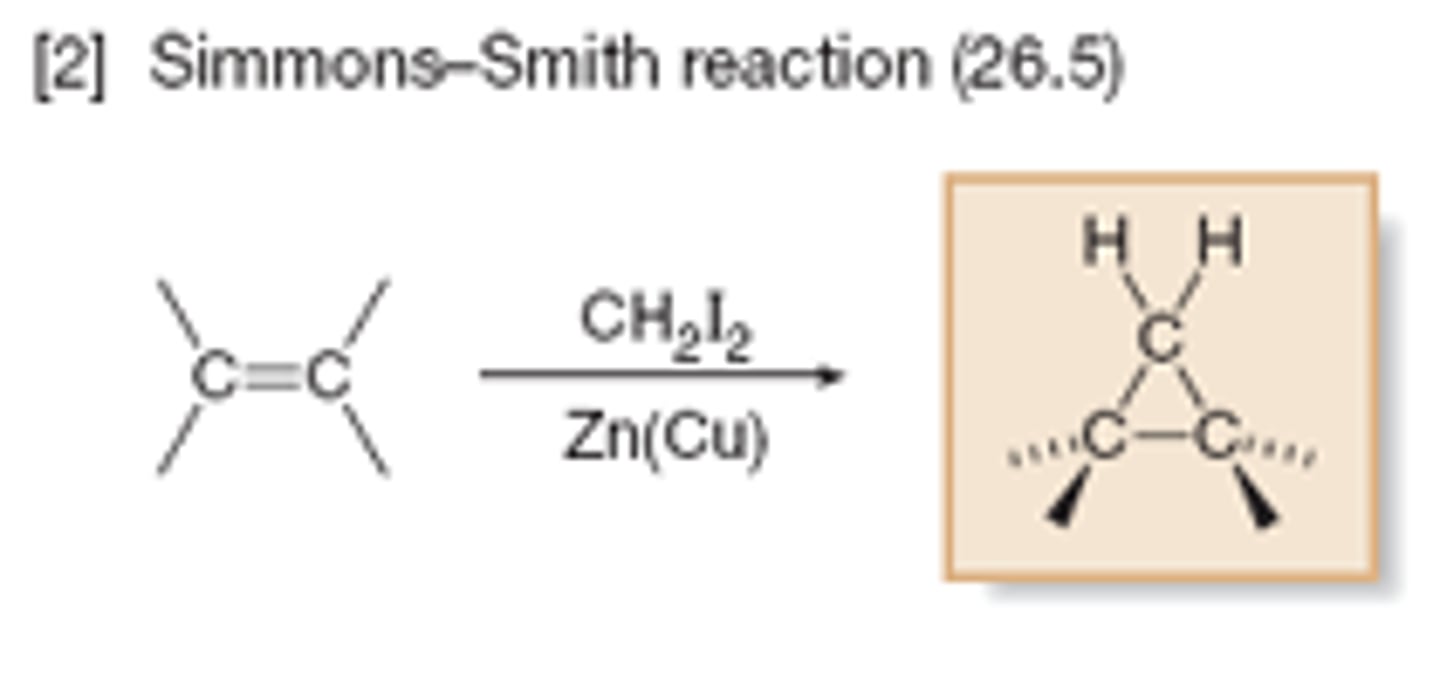
Cyclopropane synthesis using alkenes/carbenes
Alkene + carbene (sp2) --> cyclopropane
CHCl3 + OH- --> CCl2 (carbene)
Carbene: no formal charge but missing electron pair
Simmons-smith
Alkene + CH2I2 + Zn(Cu) --> cyclopropane
Alkyne hydration by oxymercuration
mark
-enol formed spontaneously convert to ketone
Alkyne reduction by Pd/Pt hydrogenation (Metal/Lindlar/Li)
H2 + metal catalyst --> alkane
H2 + lindlar catalyst --> alkene
Lindlar --> cis
Lithium --> trans
Alkyne hydration by hydroboration
Mark does not apply
Internal alkynes - both monosubstituted so no regioselectivity, forms enol —> ketone
Terminal alkynes - adds OH to the less bulky end, forms enol —> aldehyde
Alkyne synthesis by elimination
2 dehydrohalogenation reaction of alkane to form alkyne
Or
1 dehydrohalogenation reaction with strong base of alkene to form alkyne