chemistry 2 ch10 liquids and solids
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
31 Terms
In terms of their bulk properties, how do liquids and solids differ? How are they similar?
Liquids and solids are similar in that they are matter composed of atoms, ions, or molecules. They are incompressible and have similar densities that are both much larger than those of gases. They are different in that liquids have no fixed shape, and solids are rigid.
What is the evidence that all neutral atoms and molecules exert attractive forces on each other?
All atoms and molecules will condense into a liquid or solid in which the attractive forces exceed the kinetic energy of the molecules, at sufficiently low temperature.
Define and give example of dipole‑dipole attraction
A dipole-dipole attraction is a force that results from an electrostatic attraction of the positive end of one polar molecule for the negative end of another polar molecule (e.g., ICI molecules attract one another by dipole-dipole interaction).
Define hydrogen bond
A hydrogen bond is when a hydrogen atom is bonded to one of the more electronegative atoms, such as a fluorine, oxygen, nitrogen, or chlorine atom. The electrostatic attraction between the partially positive hydrogen atom in one molecule and the partially negative atom in another molecule gives rise to a strong dipole-dipole interaction.
Why do the boiling points of the noble gases increase in the order He < Ne < Ar < Kr < Xe?
The London forces typically increase as the number of electrons increases.
Arrange the following set of compounds in order of increasing boiling point temperature: HCl, H2O, SiH4
SiH4 < HCl < H2O
explanation: SiH4 is nonpolar, weakest forces usually london
HCl polar, stronger dipole dipole
water is the strongest due to polarity and h bonds
finding polarity
difference in electronegativity
difference > 2, ionic/steals electron
1.7 > difference > 0.5, polar covalent forms
difference < 0.5, nonpolar covalent
Arrange the following set of compounds in order of increasing boiling point temperature: O2, NO, N2?
N2 < O2 < NO
explanation: higher boiling point due to higher mass and polarity
both nonpolar but o higher molar mass
NO: polar
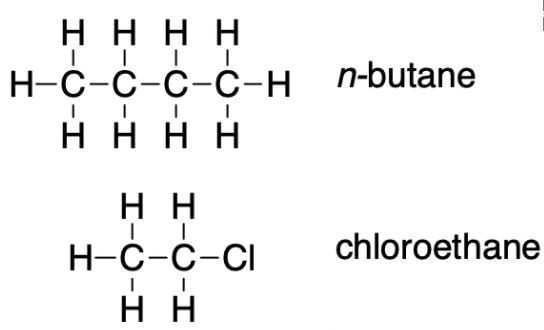
On the basis of intermolecular attractions, explain the differences in the boiling points of n–butane (–1 °C) and chloroethane (12 °C), which have similar molar masses. (image)
Only rather small dipole-dipole interactions from C-H bonds are available to hold n-butane in the liquid state. Chloroethane, however, has rather large dipole interactions because of the Cl-C bond; the interaction, therefore, is stronger, leading to a higher boiling point.
Explain why a hydrogen bond between two water molecules is weaker than a hydrogen bond between two hydrogen fluoride molecules.
The hydrogen bond between two hydrogen fluoride molecules is stronger than that between two water molecules because the electronegativity of F is greater than that of O. Consequently, the partial negative charge on F is greater than that on O. The hydrogen bond between the partially positive H and the larger partially negative F will be stronger than that formed between H and O.
remember: electronegativity Fluorine→ Oxygen→ Nitrogen→ Chlorine
Identify ALL the intermolecular forces present in CH3CH2CH3 (There could be more than one answer):
dispersion because nonpolar
Identify ALL the intermolecular forces present in CH3CH2OH (There could be more than one answer):
dispersion forces, hydrogen bonding, dipole-dipole attraction
explanation: london always present
dipole-dipole because polarity between o, c and h,
hydrogen bonding from o and h
Identify ALL the intermolecular forces present in CH3CH2Cl (There could be more than one answer)
dispersion forces, dipole-dipole attraction
explanation: dispersion in CH3/CH2
dipole-dipole is in Cl-C/polar
Although steel is denser than water, a paper clip or steel needle placed carefully lengthwise on the surface of still water can be made to float (image). Explain at a molecular level how this is possible.
The water molecules have strong intermolecular forces of hydrogen bonding. The water molecules are thus attracted strongly to one another and exhibit a relatively large surface tension, forming a type of “skin” at its surface. This skin can support a bug or paper clip if gently placed on the water.

The surface tension and viscosity of water at several different temperatures are given in the table below. As temperature increases, what happens to the surface tension of water? Explain why this occurs, in terms of molecular interactions and the effect of changing temperature.
As the water reaches higher temperatures, the increased kinetic energies of its molecules are more effective in overcoming hydrogen bonding, and so its surface tension decreases. Surface tension and intermolecular forces are directly related.
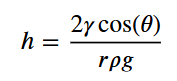
Water rises in a glass capillary tube to a height of 17 cm. What is the diameter of the capillary tube?
h=(height)
y=surface tension (water)= 0.0728 N/m
0=contact angle (on glass is 0)
p= density= for water 1000kg/m³
gravity= 9.8 m/s²
1.7 × 10^-4 m
What predominate (strongest) intermolecular force is in HCl?
dipole-dipole
What predominate intermolecular force is in NH3?
hydrogen bonding
What predominate intermolecular force is in CO2?
london forces
What predominate intermolecular force is in CH3OH?
hydrogen bonding
What predominate intermolecular force is in CH3CH2CH3?
london forces
Which of the following halogens would have stronger intermolecular forces?
Br², I², F², Cl²
i²
explanation: all london dispersion, but stronger for increased molecular size, mass, electron number
periodic trend as moves down increases
Which of the following molecules would have weaker intermolecular forces?
H2O, NH3, HCl, I2
I², nonpolar
In terms of the kinetic molecular theory, in what ways are liquids similar to gases? In what ways are liquids different from gases?
They are similar in that they are made of atoms or molecules that are free to move from one position to another. They differ in that the particles of a liquid are confined to the shape of the vessel in which they are placed. In contrast, a gas will expand without limit to fill the space into which it is placed.
Define a dispersion force and give an example of a dispersion force
Dispersion forces occur as an atom develops a temporary dipole moment when its electrons are distributed asymmetrically about the nucleus. This structure is more prevalent in large atoms such as argon or radon. A second atom can then be distorted by the appearance of the dipole in the first atom. The electrons of the second atom are attracted toward the positive end of the first atom, which sets up a dipole in the second atom. The net result is rapidly fluctuating, temporary dipoles that attract one another (e.g., Ar).
Arrange the following set of compounds in order of increasing boiling point temperature: F2, Cl2, Br2
F2 < Cl2 < Br2
heaviest and larger
Arrange the following set of compounds in order of increasing boiling point temperature: CH4, C2H6, C3H8?
CH4 < C2H6 < C3H8
explanation: propane highest bc molecular mass
The melting point of H2O(s) is 0 °C. Would you expect the melting point of H2S(s) to be –85 °C, 0 °C, or 185 °C? Explain your answer.
the melting point of H2S(s) is –85 0C, because water has stronger hydrogen bonds, so it melts at a higher temperature. While H2S molecules have dipole-dipole attractions
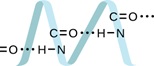
Proteins are chains of amino acids that can form in a variety of arrangements, one of which is a helix. What is the predominant (strongest) intermolecular force responsible for holding the protein strand in this shape? From the protein image below, locate the molecular points where this intermolecular force hold the protein together?
H-bonding is the principle intermolecular force holding the protein strands together. The molecular points where this intermolecular force holds the protein together is between the hydrogen in the N-H bond and the oxygen in the C = O bond.
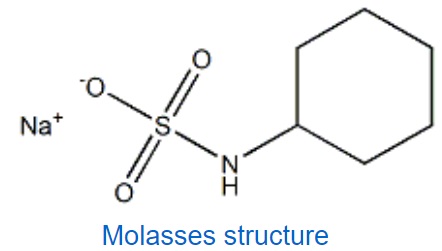
You may have heard the figure of speech “slower than molasses in winter” to describe a process that occurs slowly. Explain the reason behind such phrase, using concepts of molecular size and shape, molecular interactions, and the effect of changing temperature based on its structure shown below
The lower the temperature, the lower the kinetic energy of the molecules, the stronger the intermolecular forces. High intermolecular forces cause higher viscousity. Higher viscousity means slower flow rate. Furthermore, molasses’ molecules are big and dense which give rise to the strength of London forces between its molecules in addition to the other intermolecular forces such as dipole-dipole interactions and H-bondings.
Water rises in a glass capillary tube to a height of 0.084 m. What is the diameter of the capillary tube?
3.6´10-4 m