Chem II - Chap 11
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
In a solution this is the one in a larger amount
solvent
In a solution this is the one in a smaller amount
solute
Ablilty for substances to mix depends on two factors:
they like to be mixed or IMFs make them mix
an electrolyte solution is when the _____ are moving around in the solution
electrons
Electrolytes have a ______ boiling point
higher
A substance that dissociates into ions when dissolved in water
electrolyte
may dissolve in water, but it does not dissociate into ions when it does
non electrolyte
an electrolyte works because it dissociates into _____ that releases eletrons to charge the water
ions
electrolytes are made from _____ , ______ , and _____
ionic compounds, strong acids, weak acids
maximum amount of solute that can dissolve in a given amount of solvent at a given temperature
solubility
maximum amount of solute in the solvent
saturated solution
a solution that can store more solute
unsaturated solution
a solution that stores more than the saturated solution at a higher temperature
supersaturated solution
liquids that mix all in are
miscible
liquids that do not mix all in are
immiscible
Liquid/Liquid solubility: polar dissolves only
polar
Liquid/Liquid solubility: non polar dissolves only
non polar
the _____ the gas the more soluble it will be in water
larger
Gas/Liquid solubility: The _____ the gas the more soluble it will be in water
heavier
Cg
solubility of the gas
kh
henry law constant
Pg
pressure of gas
Cg=khPg
Henry’s law
solubility of gas in liquid decreases as temperature _____
increases
Solid/Liquid solubility: solubility of solids increases as temperature _____-
increases
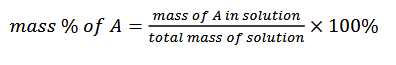
mass percentage
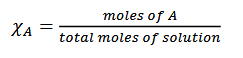
mole fraction
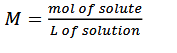
molarity
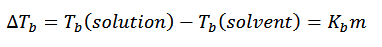
boiling point elevation
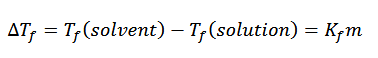
freezing point depression
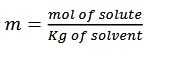
molality
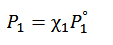
vapor pressure lowering
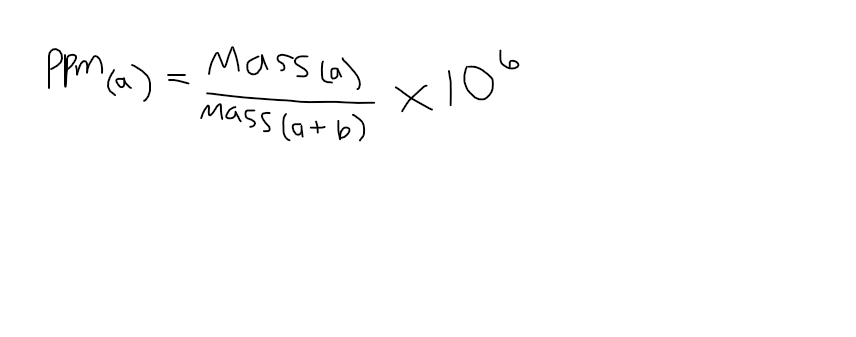
parts per million
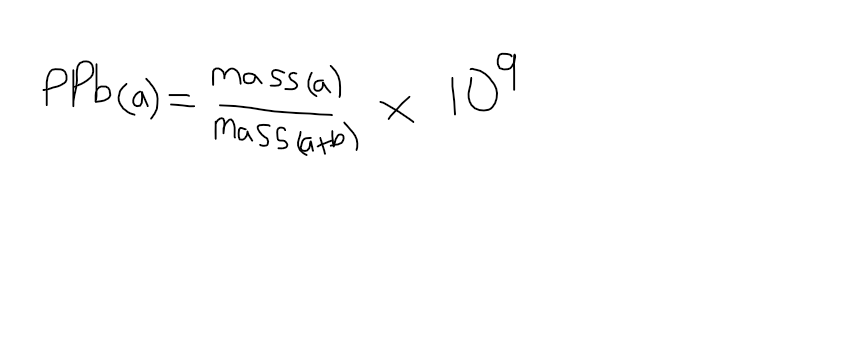
parts per billion
Strong IMFs and high boiling point
low vapor pressure
weak IMFs and low boiling point
high vapor pressure