experimental design
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
gas syringe
collect and measure the volume of gas in cm3
electronic balance
to measure mass in grams (usually copy all the numbers on the scale)
stopwatch
to measure time (usually recorded to nearest second)
thermometer
to measure temperature (to 10pm, .0 or .5)
dropper
to transfer small amounts of liquid
measuring cylinder
to measure liquid (to nearest cm3)
pipe the
to accurately measure out a fixed volume of liquid (25.0cm3, 20.0cm3)
burette
to accurately measure out a volume of liquid (to 2dp)
gases insoluble in water
hydrogen, helium, methane, carbon monoxide, nitrogen
gases soluble in water
ammonia, hydrogen chloride, nitrogen dioxide, sulfur dioxide, chlorine
slightly soluble: oxygen, carbon dioxide
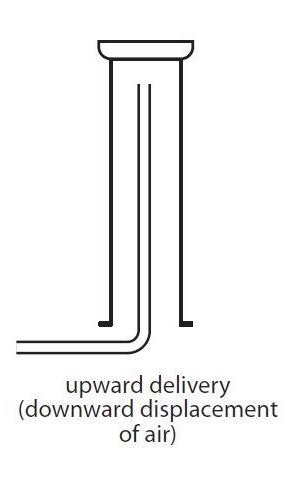
collection of gas less dense than air
upward delivery
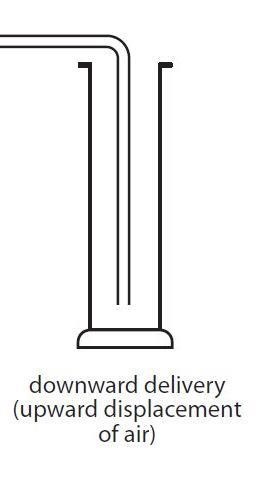
collection of gas denser than air
downward delivery
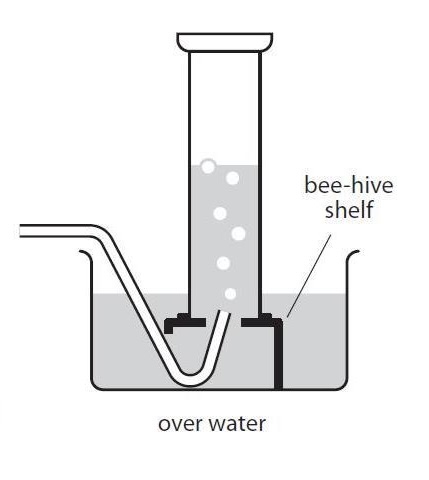
collection of gas insoluble in water
displacement of water
drying agents
concentrated sulfuric acid, quicklime (calcium oxide), fused calcium chloride
concentrated sulfuric acid
dry most gases except ammonia, acidic in nature so cannot dry alkali gases
quicklime (calcium oxide)
alkaline in nature, cannot dry acidic gas, used to dry ammonia
fused calcium chloride
dry most gases except ammonia, neutral
independent variable
the variable you change
dependent variable
the variable you measure
controlled variable
the variables kept the same