colours of transition metals
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
why are the colours of different transition metal complexes different?
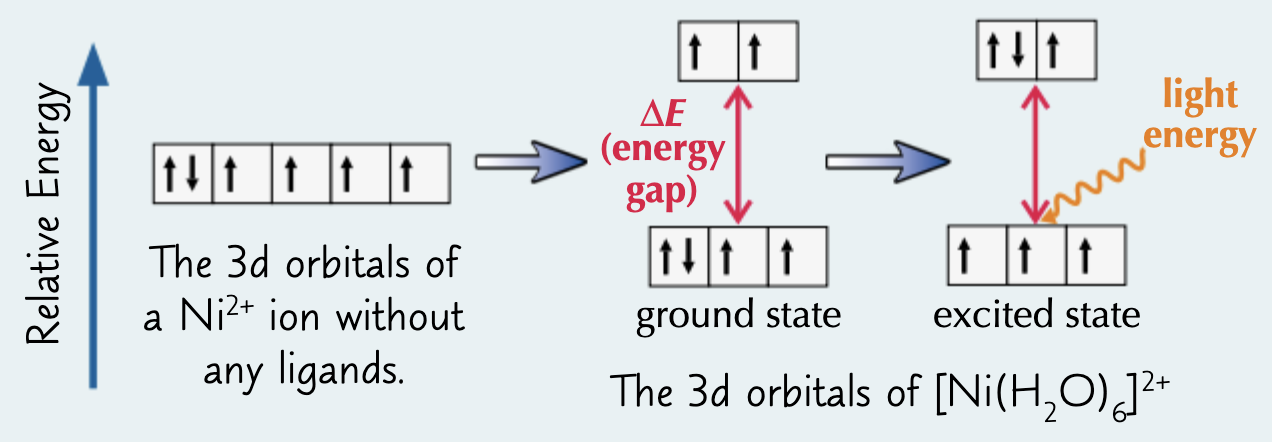
when a ligand bonds to a transition metal, the d orbitals split in E
when they absorb visible light, the e- move up to higher E orbitals i.e. from the ground state to an excited state - this is called excitation
the difference in E is called ΔE
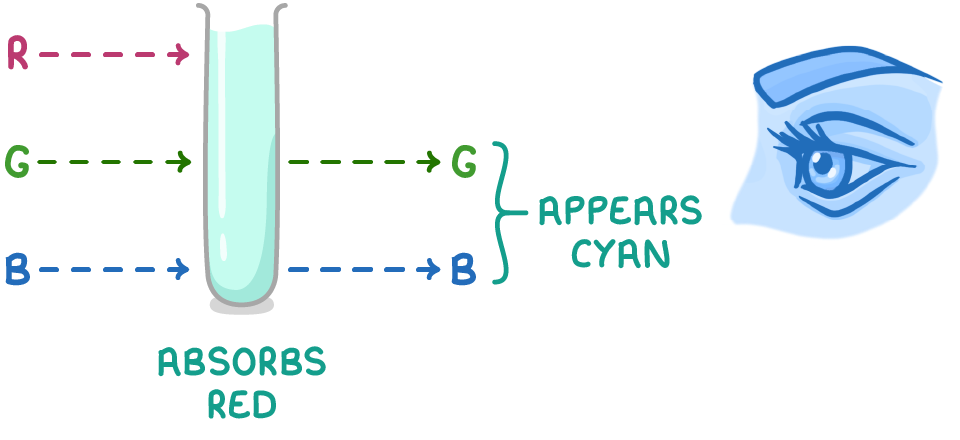
remaining wavelengths of light are transmitted/reflected by the complex and combine to form the colour, which is complementary to the wavelengths absorbed
give the eqn for calculating the difference in E and state what each symbol corresponds to:
ΔE = h ν = hc / λ, where:
ΔE: energy absorbed / J
h: Planck’s constant = 6.63 × 10-34 Js
v (Greek letter nu): freq of light absorbed / Hz
c: speed of light = 2.998 × 108 ms-1
λ: wavelength of light absorbed / m
give 3 factors which might affect the colour of a transition metal complex:
change in oxidation state
change in coordination number
change in ligand
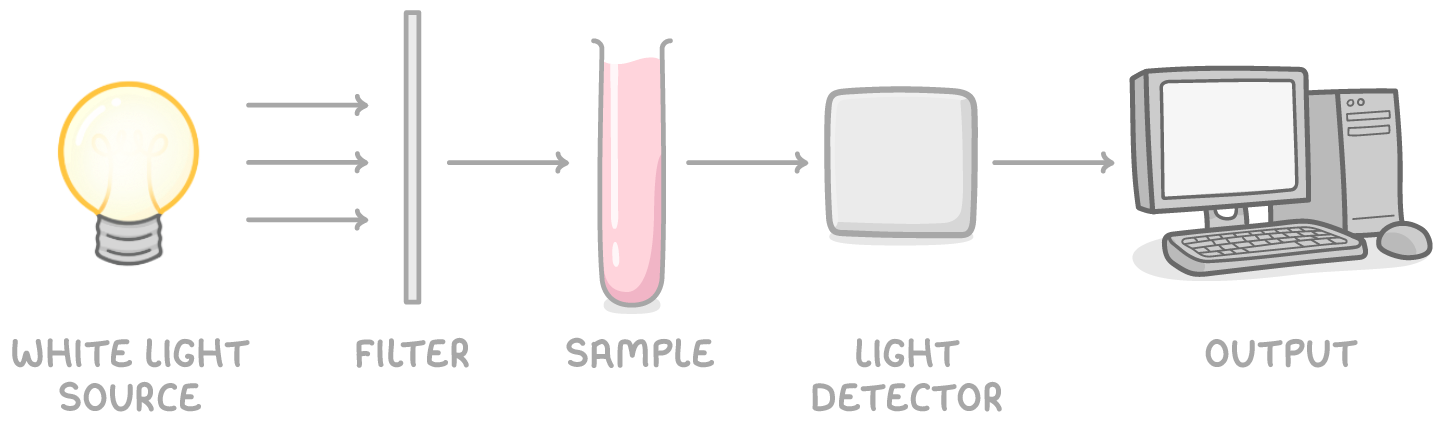
how can we use colorimetry to determine the concentration of transition metal ions in a solution of unknown concentration?
add an appropriate ligand to intensity colour
make up solutions of known concentrations and measure absorption/transmission
plot calibration curve
measure absorption of unknown and use calibration curve to deduce concentration