Alchohols, Phenols and Ethers
1/51
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
Alcohols and Phenols
Alcohols and phenols are formed when a hydrogen atom in a hydrocarbon, aliphatic and aromatic respectively, is replaced by –OH group.
Name a substance that can be used as an antiseptic as well as a disinfectant
Phenol : 0.2% solution of phenol is an antiseptic while 2% solution is used as disinfectant.
Classification
i) Based on Number of OH groups
Monohydric Alcohols/Alkanols
Dihydric Alcohols/Diols
Polyhydric Alcohols
ii) Based on Molecular Structure
Alkyl Alcohols
Both α and β carbon are single-bonded
R-CH₂OH
Allyl Alcohols
β carbon is double-bonded
CH₂=CH-C(R)(R’)-OH
Benzyl Alcohols
α carbon is attached to the benzene ring/β carbon is part of the benzene ring
C₆H₆-CH₂OHVinyl Alcohols
α carbon is double-bonded
CH₂=CH-OH
Anyl Alcohols
OH is attached to benzene
C₆H₅OH
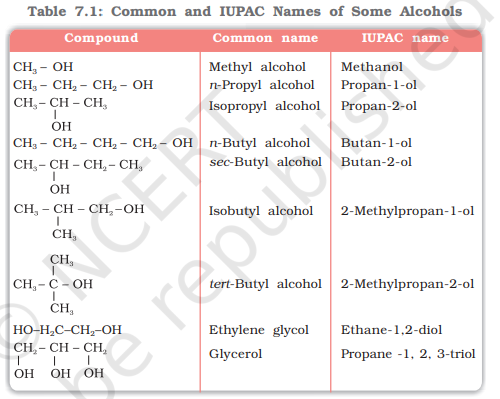
Common and IUPAC Names of Some Alcohols
Preparation of Alcohols
From alkenes
From carbonyl compounds
From Grignard reagents
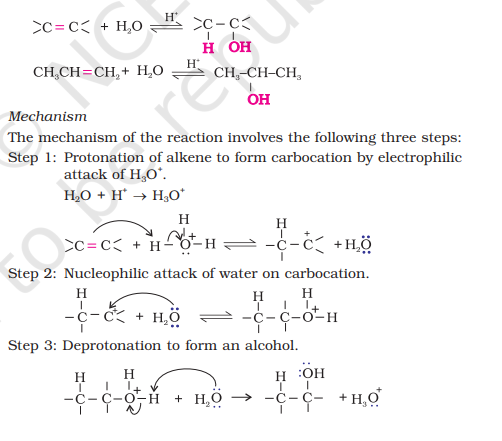
1.1) From Alkenes - acid catalysed hydration
i) How do you convert Propene to propane-2-ol?
ii) How do you convert 2-methyl-1-pentene to 2-methyl pentan-2-ol?
iii) Show how will you synthesize 1-phenylethanol from a suitable alkene.
markonikov’s addition
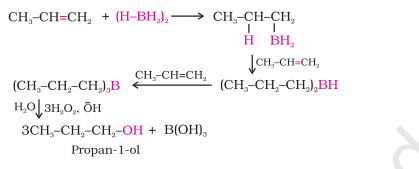
1.2) From Alkenes - By hydroboration–oxidation
insert how last step works
From Carbonyl Compounds (compounds with C=O) and Carboxylic Acids
i) How do you convert Primary alcohol to carboxylic acid?
ii) How do you convert Butan-2-one to Butan-2-ol?
iii) How do you convert ethanol to ethanal?
iv) Draw the structure and name of the product formed if the following alcohols are oxidized:
a) CH₃CH₂CH₂CH₂OH b) 2-butenol c) 2-methyl-1-propanol
Assume that an excess of oxidising agent is used.
1° Alcohol → [O (removal of hydrogen)] Aldehyde → [O (addition of oxygen)] Carboxylic Acid
R-OH → [O] R-CHO → [O] R-COOH
Carboxylic Acid → [H (removal of oxygen)] 1° Alcohol and Aldehyde → [H (addition of hydrogen)] 1° Alcohol
R-COOH → [H] R-OH and R-CHO → R-OH
carboxylic acids cannot directly be reduced to aldehydes
2° Alcohol → [O (removal of hydrogen)] Ketone
R-CH(OH]-R’ → [O] R-C(O)-R’
Ketone → [H (addition of hydrogen)] 2° Alcohol
R-C(O)-R’ → [H] Ketone
2) From Carbonyl Compounds
i) By reduction of aldehydes and ketones:
RCHO → [H-Pd] RCH₂OH
RCOR’ → [NaBH₄] R-CH(OH)-R’
ii) By reduction of carboxylic acids and esters:
RCOOH → [LiAlH₄,H₂O] RCH₂OH
However, LiAlH4 is an expensive reagent, and therefore, used for preparing special chemicals only. Commercially, acids are reduced to alcohols by converting them to esters, followed by their reduction using hydrogen in the presence of a catalyst (catalytic hydrogenation).
reaction in image
insert explanation here
![<p>i) By reduction of aldehydes and ketones:</p><p>RCHO → [H-Pd] RCH₂OH</p><p>RCOR’ → [NaBH₄] R-CH(OH)-R’</p><p>ii) By reduction of carboxylic acids and esters:<br><br>RCOOH → [LiAlH₄,H₂O] RCH₂OH</p><p>However, LiAlH4 is an expensive reagent, and therefore, used for preparing special chemicals only. Commercially, acids are reduced to alcohols by converting them to esters, followed by their reduction using hydrogen in the presence of a catalyst (catalytic hydrogenation).<br><br><em>reaction in image</em><br><em>insert explanation here</em></p>](https://knowt-user-attachments.s3.amazonaws.com/51874ff1-c664-44b1-8911-85476768486e.png)
3) From Grignard Reagents
i) Give the structure and IUPAC name of the product formed when propanone is reacted with methyl magnesium bromide followed by hydrolysis.
ii) How are the following alcohols prepared by the reaction of a suitable grignard reagent on methanal
a) 2-Methly Propanol b) C6H5CH2OH
Formaldehyde → [Grignard Reagent, H3O+] 1° Alcohol
Aldehyde → [Grignard Reagent, H3O+] 2° Alcohol
Ketone → [Grignard Reagent, H3O+] 3° Alcohol
![<p>Formaldehyde → [Grignard Reagent, H3O+] 1° Alcohol</p><p>Aldehyde → [Grignard Reagent, H3O+] 2° Alcohol</p><p>Ketone → [Grignard Reagent, H3O+] 3° Alcohol</p>](https://knowt-user-attachments.s3.amazonaws.com/17ac69b1-f721-4d4d-bd33-1eb7fc30348c.png)
4) From Alkyl Halides (Nucleophilic Substitution SN2 Mechanism)
i) Show how will you synthesize:(i) cyclohexylmethanol using an alkyl halide by an SN2 reaction
(ii) pentan-1-ol using a suitable alkyl halide?
R-Cl + NaOH → R-OH + NaCl
Preparation of Phenols
From haloarenes
From benzenesulphonic acid
From diazonium salts
From cumene
1) From Haloarenes (Dow’s Process)
i) How do you convert chlorobenzene into phenol?
note has simpler reaction
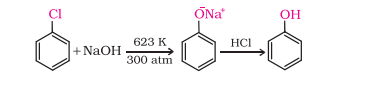
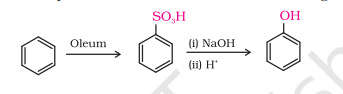
2) From Benzenesulphonic Acid
i) You are given benzene, conc. H2SO4 and NaOH. Write the equations for the preparation of phenol using these reagents.
Oleum - fuming sulphuric acid
H2S2O7 (or) H2SO4(SO3) (or) H2SO4 heating
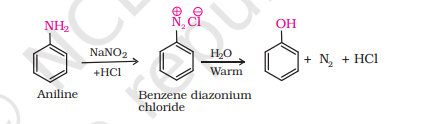
3) From Diazonium Salts
i) How do you convert Aniline to phenol?
note has simpler reaction
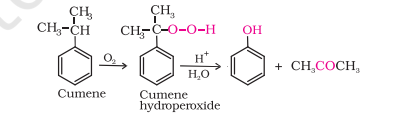
4) From Cumene
note has simpler reaction
Physical Properties
The boiling point of alcohols and phenols increases with the increase in molecular mass due to an increase in van der Waal’s forces
Isomeric alcohols show a decrease in boiling point with an increase in branching. This is because branching decreases surface area which in turn decreases van der Waal’s forces
Alcohols have higher boiling points than ethers, haloalkanes and hydrocarbons of comparable masses due to the presence of intermolecular hydrogen bonding
Alcohols are soluble in water as they form hydrogen bonds with the water molecules. Their solubility decreases with an increase in molecular size. This is due to an increase in the size of the hydrophobic alkyl group.
Order of Boiling Point
carboxylic acids > alcohols > amines }- Hydrogen Bonding
> ketones > aldehydes}- Strong dipole dipole interactions
> ethers > haloalkanes }- Weak dipole dipole interactions
> hydrocarbons }- Van der waal’s forces
always write answers for B.P questions in terms of the one with higher B.P
Acidic Character of Alcohols and Phenols
i) Give two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of ethanol.
Alcohol is less acidic than water due to the presence of an electron-donating alkyl group (+I effect), the electron density of oxygen increases which decreases the ability to donate a proton
The acidic nature of alcohols is due to the polar nature of O-H bond
Phenols are more acidic than water because phenoxide ion is stabilized by resonance.
ROH < H₂O < C₆H₅OH
Among 1°, 2°, and 3° alcohols, 1° alcohol > 2° alcohol > 3° alcohol because 3° has more +I = more electron density.
Among substituted phenols or phenol derivatives, phenols with electron-withdrawing groups are more acidic than phenols with electron-donating groups.
phenol (e.w.g) > phenol > phenol (e.d.g)
The acidic strength of phenol is more pronounced when an e.w.g is present at ortho and para positions. This is due to the efffective delocalisation of negative charge in phenoxide ion when substituent is at ortho or para position
para > ortho > meta
Order of Acidic Character
3° Alcohol < 2° Alcohol < 1° Alcohol < H₂O < phenol (e.d.g) < phenol < phenol (e.w.g)
Chemical Properties of Alcohols
a) Reactions involving cleavage of O–H bond
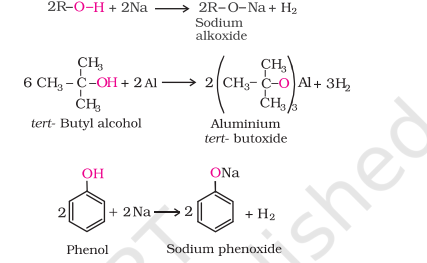
Reaction with metals
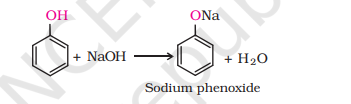
Reaction with Alkali
Reaction with Grignard Reagent
Esterification
1) Reaction with Metals
2) Reaction with Alkali
R-OH + NaOH → No reaction
3) Reaction with Grignard Reagent
R-OH + R’MgX → R-H +Mg(X)(OR)
4) Esterification (Preparation of Esters)
i) How do you convert Propan-1-ol to 1-propoxypropane? (i think it comes under this topic)
Model 1: Alcohol + Carboxylic Acid → [conc.H₂SO₄] Ester + Water
R-OH + R’COOH → [conc.H₂SO₄] R’-C(O)-OR +H₂O
CH₃CH₂-OH + CH₃COOH → [conc.H₂SO₄] CH₃-C(O)-O-CH₂CH₃ +H₂O
Model 2: Alcohol + Acid A
Acetylation of Salicylic Acid
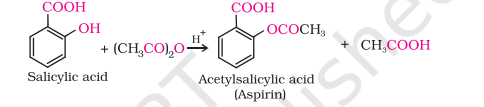
Reactions involving cleavage of carbon – oxygen (C–O) bond
Lucas Test
With Chlorinating Agents
Dehydration
Test for Phenol
Haloform Reaction
Catalytic Dehydrogenation
1) Lucas Test (Reaction with Hydrogen Halides)
i) Give a chemical test to distinguish between 2-Pentanol and 3-Pentanol
ii) Give a chemical test to distinguish between 1-Propanol and 2-Propanol.
Test is used to distinguish 1ᵒ, 2ᵒ and 3ᵒ alcohols
Depends on order of reactivity ( 3ᵒ>2ᵒ>1ᵒ )
Turbidity → Formation of precipitate
General Formula:
ROH + HX → R–X + H₂O
Reactions:
1ᵒ Alcohol:
R-CH₂-OH + HCl → [ZnCl₂] R-Cl + H₂O
No turbidity at room temperature
2ᵒ Alcohol:
R-CH(R)-OH + HCl → [ZnCl₂] R-CH(R)-Cl + H₂O
Turbidity after 5-10 minutes
3ᵒ Alcohol:
R-CH(R)(R)-OH + HCl → [ZnCl₂] R-CH(R)(R)-Cl + H₂O
Immediate Turbidity
2) With Chlorinating Agents
ROH + PCl₅ → RCl + POCl₃ + HCl
ROH + SOCl₂ → RCl + SO₂ + HCl
3ROH + PCl₃ → 3R-Cl + H₃PO₃
3) Dehydration
i) How do you convert Propan-2-ol to propane?
ii) How would you convert ethanol to ethene?
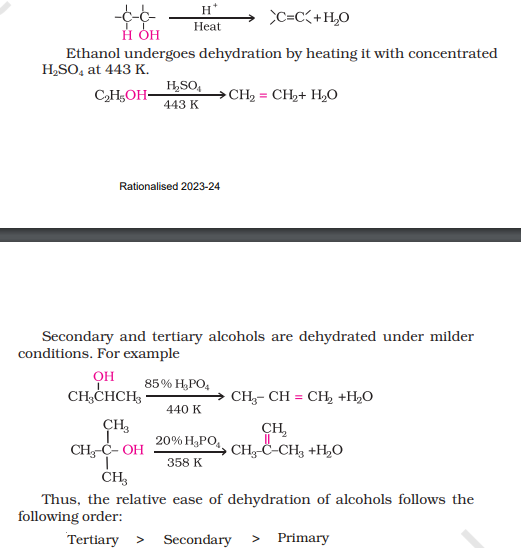
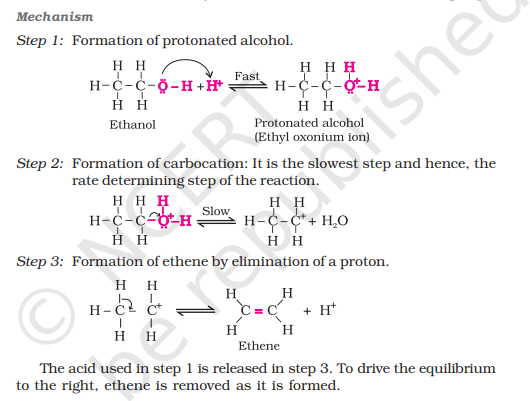
Mechanism of Dehydration of Alcohol
use note
Step 1: Protonation
Step 2: Elimination of water
Step 3: Deprotonation
4) Test for Phenol (neutral FeCl₃ Test)
i) Give a chemical test to distinguish between Benzoic acid and Phenol
ii) Give a chemical test to distinguish between Ethanol and Phenol
C₆H₅OH + FeCl₃ → (C₆H₅O)₃Fe violet precipitate + 3HCl
5) Haloform Reaction (Iodoform Test)
i) Give a chemical test to distinguish between 2-Pentanol and 3-Pentanol
ii) Give a chemical test to distinguish between 2-propanol and 2-methyl-2-propanol.
iii) Give a chemical test to distinguish between 1-Propanol and 2-Propanol.
The test only identifies alcohol with (CH₃-CH(OH)-)
CH₃-CH(OH)-R → [NaOI, NaOH + I₂] CHI₃ yellow precipitate + R-COONa
6) Catalytic Dehydrogenation (Oxidation)
i) How do you convert Propan-2-ol to propanone?
ii) How do you convert Hexan-1-ol to hexanal?
R-CH₂OH → [Cu,573K] R-CHO
R-CH(R’)-OH → [Cu,573K] R-C(R’)=O
(CH₃)C(CH₃)(CH₃)(OH) → [Cu, 573K] CH₃-C(CH₃)=CH₃
Chemical Properties of Phenol
Oxidation
Reduction
Bromination
Nitration
Reimer Tiemann Reaction
Kolbe’s Reaction
Acetylation of Salicylic Acid
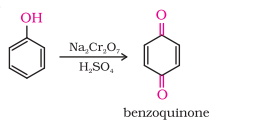
1) Oxidation
i) How do you convert Phenol to Benzoquinone?
2) Reduction (Reaction Of Phenol With Zinc Dust)
i) How do you convert Phenol to Benzene?
ii) How is toluene obtained from phenol?
iii) How would you obtain acetophenone from phenol?
C₆H₅OH → [Zn dust] C₆H₆
![<p><span>C₆H</span>₅<span>OH → [Zn dust] C</span>₆H₆</p>](https://knowt-user-attachments.s3.amazonaws.com/d26afc7d-2ffe-4fb6-81f5-b123a21777ac.png)
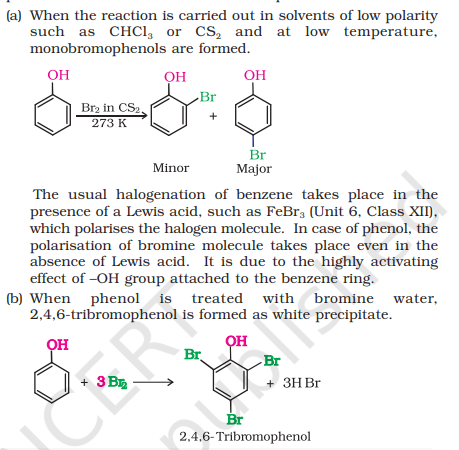
3) Bromination
i) How to convert Phenol to 2, 4, 6-tribromophenol?
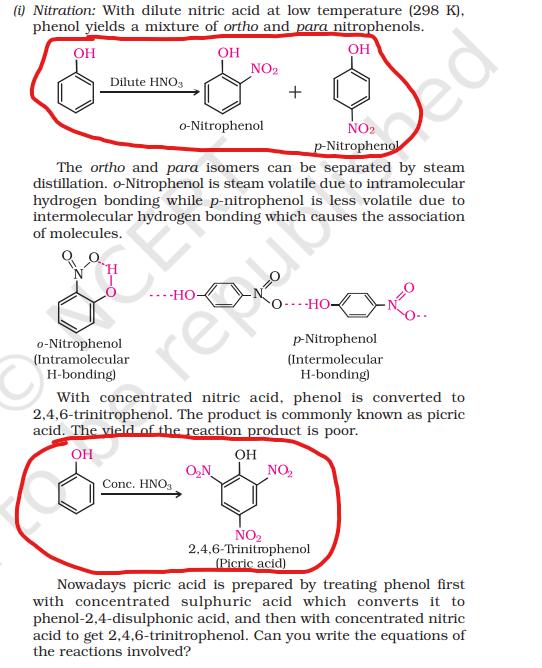
4) Nitration
i) How do you convert Phenol to 2,4,6-trinitrophenol(picric acid)?
ii) Which of the following isomers is more volatile: o-nitrophenol or p-nitrophenol?
It is named picric acid because of its bitter taste
Picric acid is used to make explosives
o-Nitrophenol is more steam volatile than p-Nitrophenol due to the presence of intramolecular
H-bonding.
p-nitrophenol shows intermolecular H–bonding.
5) Reimer Tiemann Reaction
C₆H₅OH → [CHCl₃, NaOH, H₃O⁺] C₆H₄OH CHO
![<p>C₆H₅OH → [CHCl<span>₃, NaOH, H₃O⁺] </span>C₆H<span>₄</span>OH CHO</p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/b93ad0cc-3b70-4301-9198-b88a8257301c.png)
6) Kolbe’s Reaction
C₆H₅OH → [CO₂, NaOH, H₃O⁺] C₆H₄OH COOH
![<p>C₆H₅OH → [CO₂, NaOH, H₃O⁺] C₆H₄OH COOH</p>](https://knowt-user-attachments.s3.amazonaws.com/db6d3310-feb7-4433-8936-a4b495a2f853.png)
Commercially Important Alcohols
Methanol
aka wood spirit bc it was produced by destructive distillation of wood
Can cause blindness and large quantities causes even death
Ethanol
Glucose and fructose undergo fermentation in the presence of another enzyme, zymase, which is found in yeast to form ethantol
The commercial alcohol is made unfit for drinking by mixing in it some copper sulphate (to give it a colour) and pyridine (a foul smelling liquid).
100% pure ethanol is called absolute alcohol
95% ethanol is called rectified spirit/surgical spirit/rubbing alcohol
Methylated spirit - Mixture of methanol and ethanol
Ethers
R-O-R
OR - Alkoxy group
Simple ethers - Symmetric (R-O-R)
Mixed ethers - Asymmetric (R-O-R’)
Preparation of Ethers
By Dehydration of Alcohols
Williamson Synthesis
Preparation of Ethers
1) By Dehydration of Alcohols
Can only make simple/symmetric ethers, mixed ethers cannot be prepared
The reaction follows the SN₂ mechanism (substitution) and not the elimination
Branched alkyl ethers cannot be prepared since this reaction follows SN₂ mechanism and branched alkyl ethers show more steric hindrance
Aromatic ethers cannot be prepared as they are less reactive towards nucleophilic substitution due to partial double bond character by C-O bond resonance
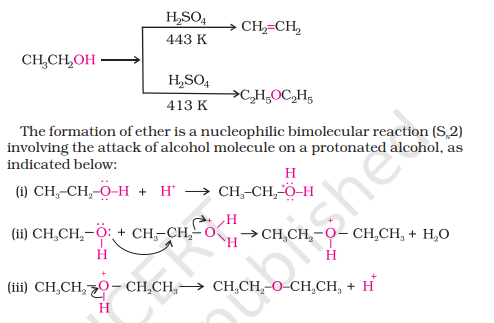
Preparation of Ethers
2) Williamson Synthesis
R-O-Na + R’-X → R-O-R’ + NaX
CH₃-CH₂-O-Na + CH₃-Cl → CH₃-CH₂-O-CH₃ + NaCl
Can make both simple and mixed ethers
Better results are obtained if the alkyl halide is primary. In case of secondary and tertiary alkyl halides, elimination competes over substitution.
Physical Properties of Ethers
The large difference in boiling points of alcohols and ethers is due to the presence of hydrogen bonding in alcohols.
The miscibility of ethers with water resembles those of alcohols of the same molecular mass.
Chemical Properties of Ethers
Reaction with HX
Electrophilic Substitution
Chemical Properties of Ethers
1) Reaction with HX
Phenol to Anisole
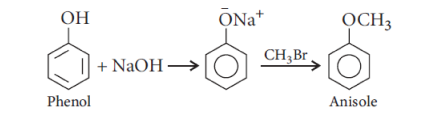
Chemical Properties of Ethers
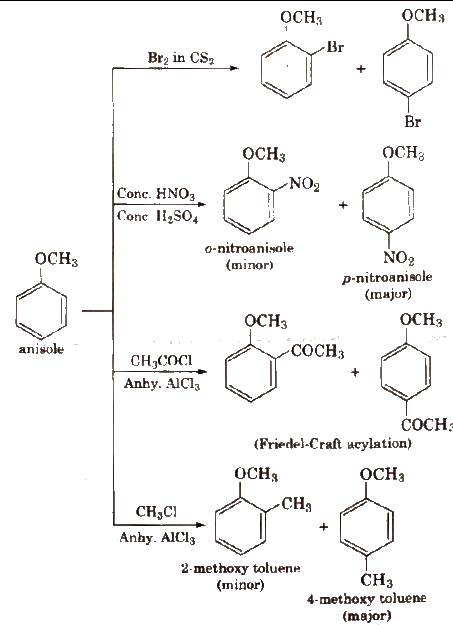
2) Electrophilic Substitution
i) How do you convert Anisole to p-bromoanisole?