Chem exam 5
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
Bond Energy
the energy needed to overcome the attraction between the nuclei and their shared electrons. The stronger the bond the higher the bond energy. is always endothermic. Takes energy to break bonds.
why is N2 and stronger bond than C2?
the size of N is smaller than C, so bond length between nitrogens is shorter
Electronegativity
the ability of an atom in a covalent bond to attract the shared electron pair to itself
order H, Cl, C, F, N, O based on decreasing EN
F> O > N = Cl > C > H
who came up with the electronegativity scale?
linus pauling
≥ 1.7 EN
mostly ionic
> 0.4 and < 1.7 EN
polar covalent
≤ 0.4 EN
nonpolar covalent
0 EN
fully covalent
unlike covalent molecules, no compound can be fully _____
ionic
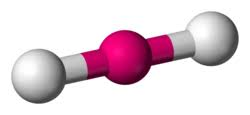
bonding group (no. and name):
bond angle:
molecular shape (class):
bonding group (no. and name): 2, linear
bond angle: 180
molecular shape (class): linear ; AX2
bonding group (no. and name):
bond angle:
molecular shape (class):
bonding group (no. and name): 3, trigonal planar
bond angle: 120
molecular shape (class): trigonal planar, AX3
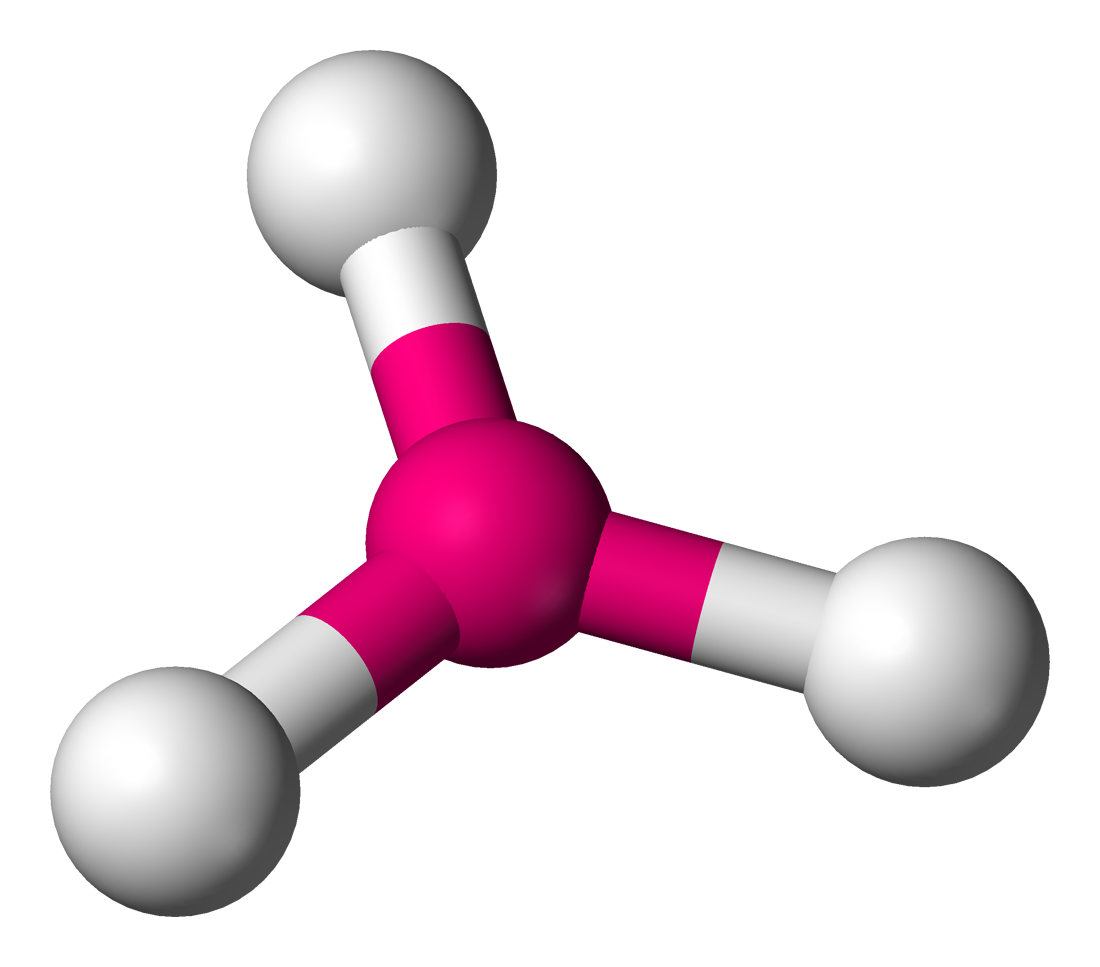
bonding group (no. and name):
bond angle:
molecular shape (class):
bonding group (no. and name): 2, trigonal planar
bond angle: <120
molecular shape (class): bent or V shaped; AX2E
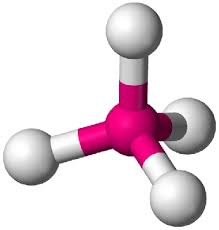
bonding group (no. and name):
bond angle:
molecular shape (class):
bonding group (no. and name): 4, tetrahedral
bond angle: 109.5
molecular shape (class): tetrahedral, AX4
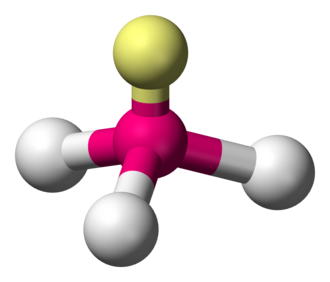
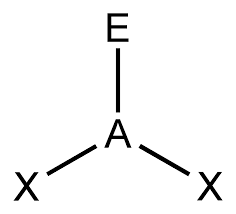
bonding group (no. and name):
bond angle:
molecular shape (class):
bonding group (no. and name): 3, tetrahedral
bond angle:<109.5
molecular shape (class): trigonal pyrimidal, AX3E
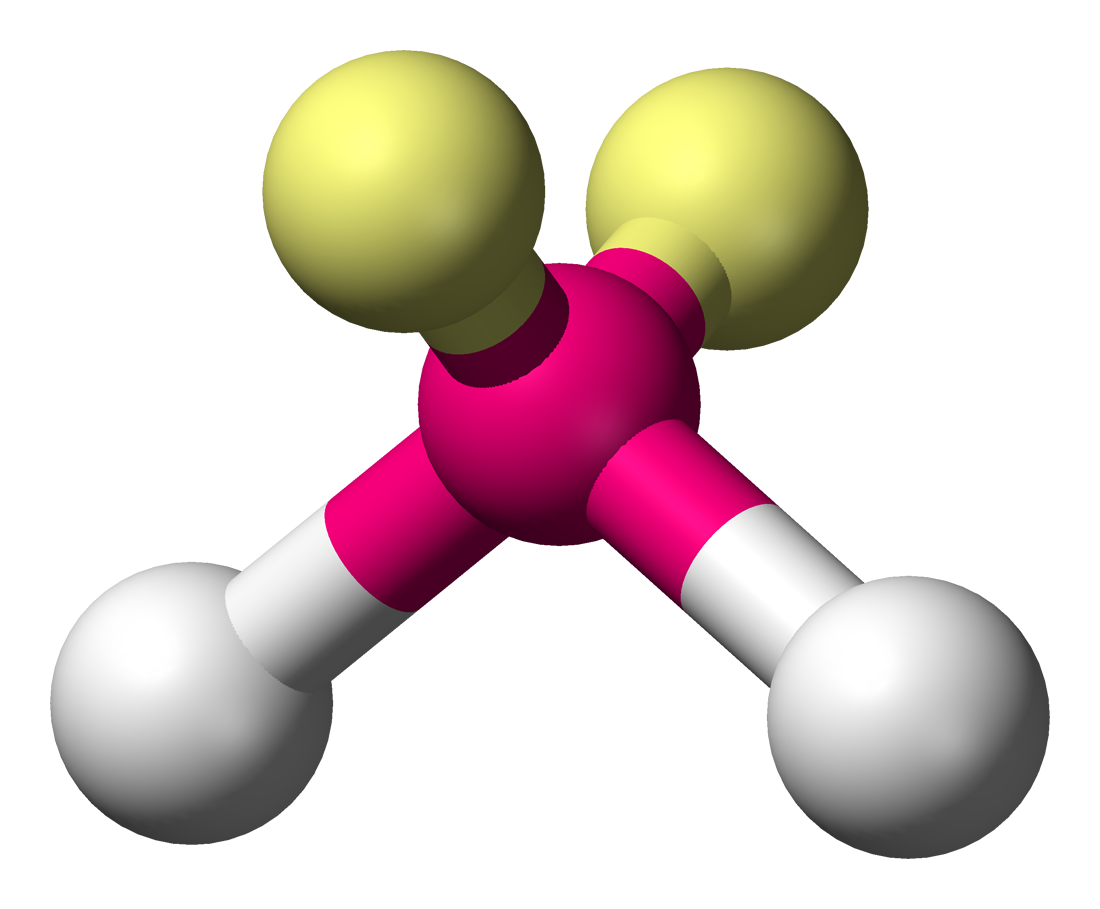
bonding group (no. and name):
bond angle:
molecular shape (class):
bonding group (no. and name): 2, tetrahedral
bond angle: <109.5
molecular shape (class): bent or V shaped, AX2E2
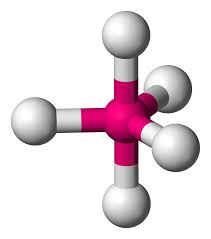
bonding group (no. and name):
bond angle:
molecular shape (class):
bonding group (no. and name): 5, trigonal bipyridmidal
bond angle: 90 (eq), 120 (ax)
molecular shape (class): trigonal bipyrimidial, AX5
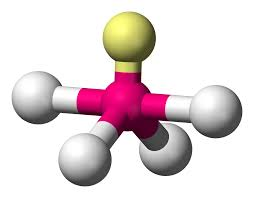
bonding group (no. and name):
bond angle:
molecular shape (class):
bonding group (no. and name): 4, trigonal bipyrimidal
bond angle: <90 , <120
molecular shape (class): seesaw , AX4E
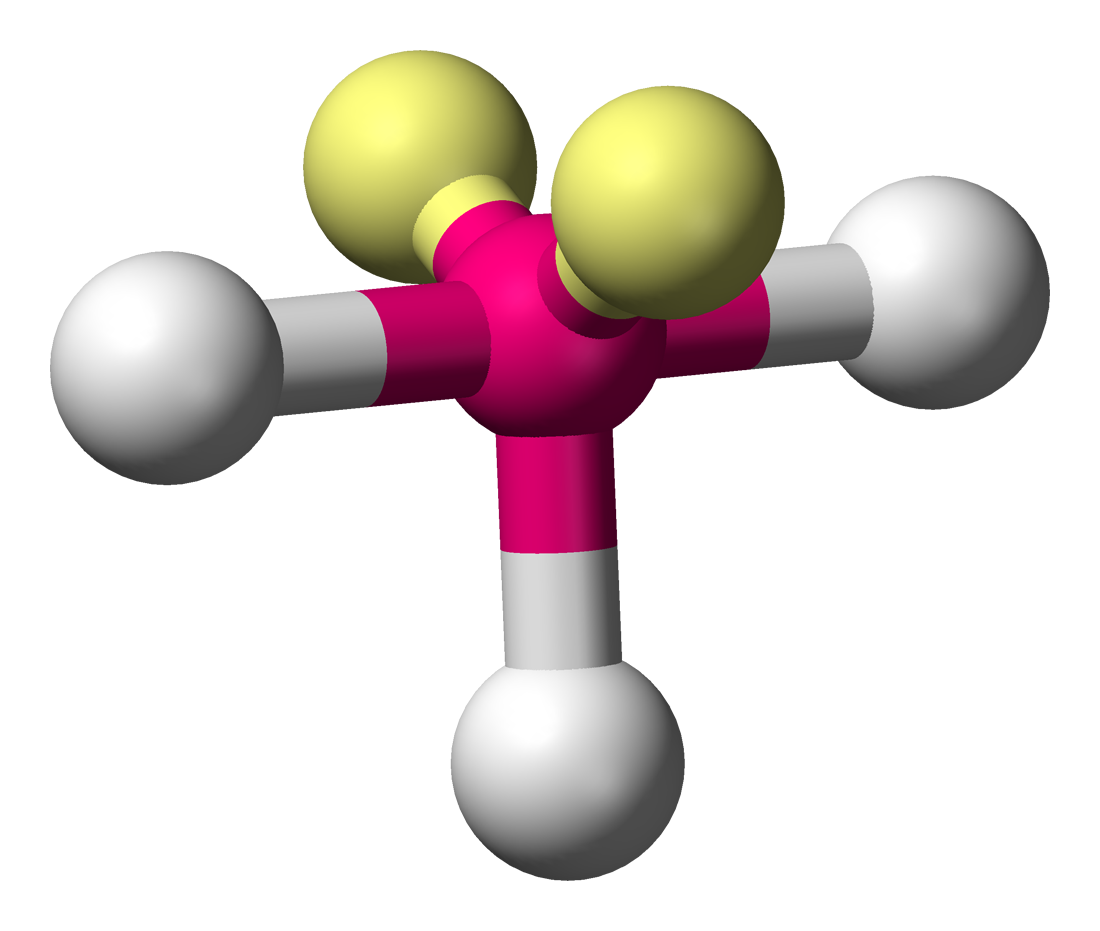
bonding group (no. and name):
bond angle:
molecular shape (class):
bonding group (no. and name): 3, trigonal bipyrimidal
bond angle: <90
molecular shape (class): T-shape, AX3E2
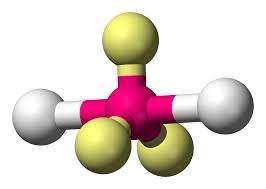
bonding group (no. and name):
bond angle:
molecular shape (class):
bonding group (no. and name): 2, trigonal bipyrimidal
bond angle: 180
molecular shape (class): linear , AX2E3
bonding group (no. and name):
bond angle:
molecular shape (class):
bonding group (no. and name): 6, octahedral
bond angle: 90
molecular shape (class): octahedral, AX6
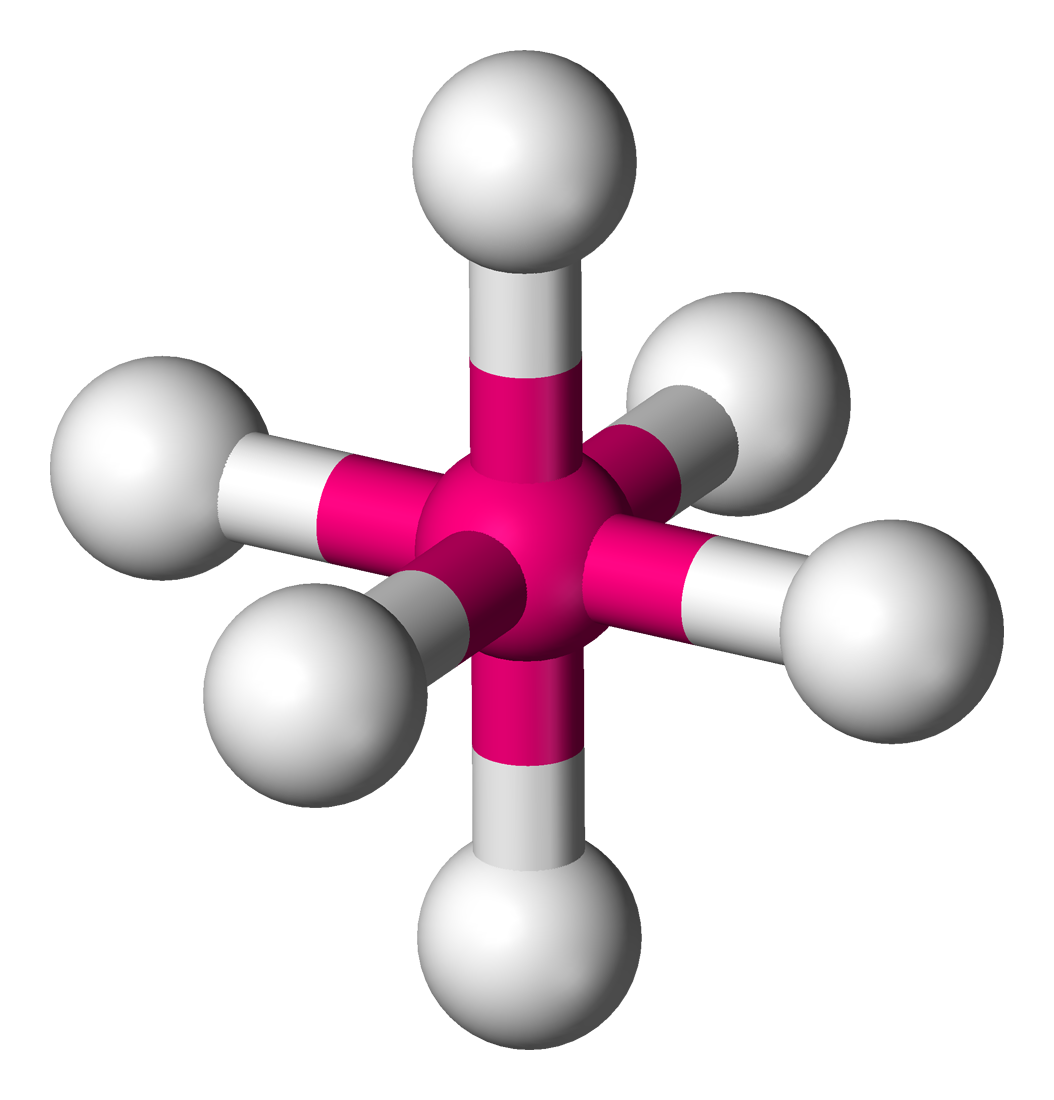
bonding group (no. and name):
bond angle:
molecular shape (class):
bonding group (no. and name): 5, octahedral
bond angle: <90
molecular shape (class): square pyrimidal, AX5E
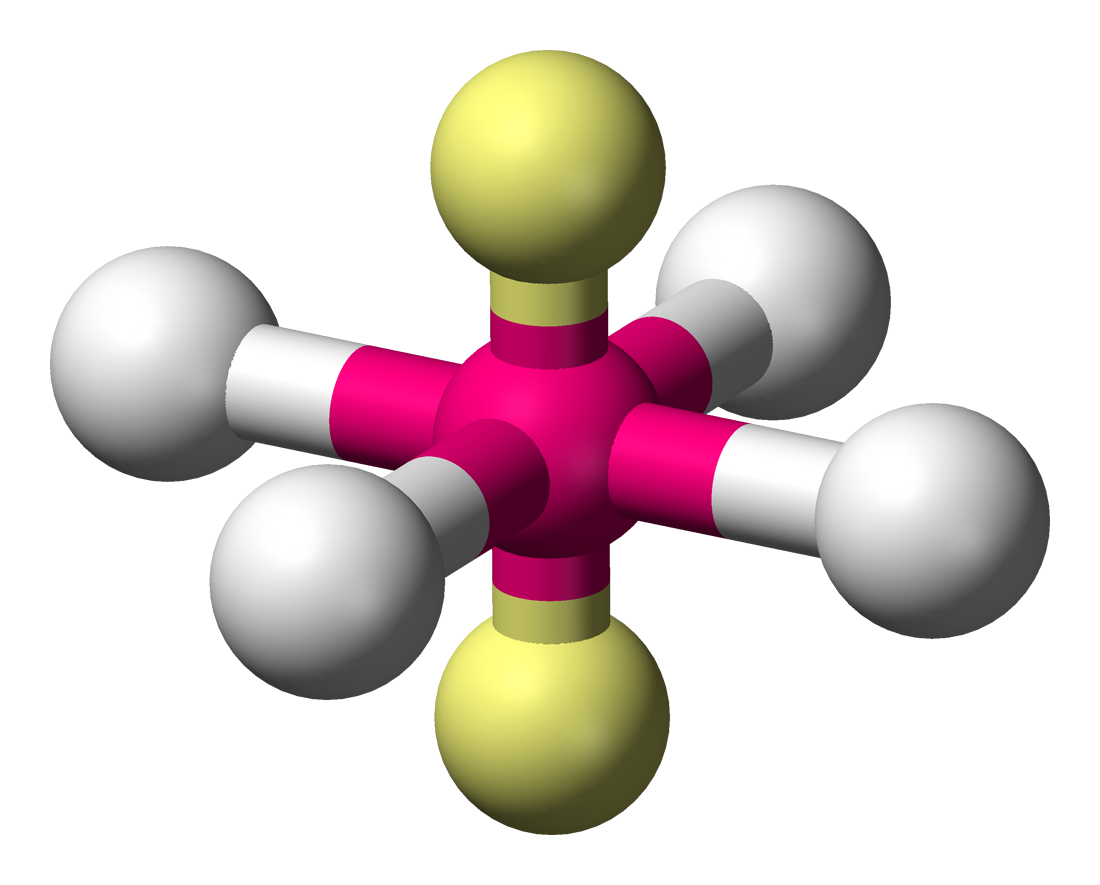
bonding group (no. and name):
bond angle:
molecular shape (class):
bonding group (no. and name): 4, octahedral
bond angle: 90
molecular shape (class): square planar, AX4E2