Lesson 28-30
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
What is the difference between kinetic and thermodynamic product?
The thermodynamic product is always the most stable product. It might take longer to form but it will be the most stable form.
The kinetic product is the product that is the fastest formed. There is a low activation energy to form it so it forms quickly.
Chat good explanation…
If a reaction is irreversible, once a product forms, it’s “locked in” — there’s no chance to go back and try another path. That means the reaction will favor the kinetic product, because it forms fastest.
If a reaction is reversible, the product that is less stable can revert to reactants, and the reaction can proceed again to form the more stable product.
So over time, the equilibrium shifts toward the thermodynamic product — the one that’s lowest in free energy.
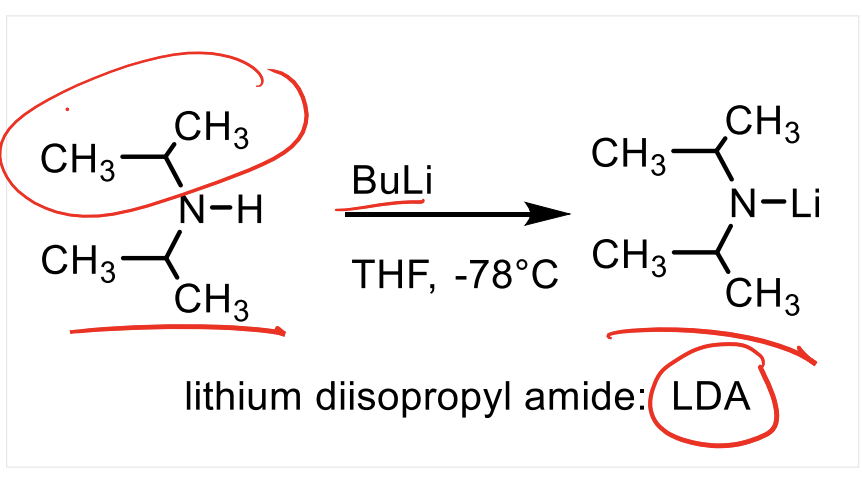
What is the pKa of the conjugate acid for LDA? What does this mean?
The pKa of the conjugate acid of LDA is 36. This means it is a really weak acid which means LDA is a really strong base.
LDA is very sterically hindered so it won’t do SN2 it will only do acid base. NH2- for example comes from the conjugate acid ammonia with a pKa of 36, but it is not sterically hindered so it will just attack the ketone rather than deprotonate the alpha hydrogen.
Explain the structure of LDA and its properties…
LDA is a very strong base and a very poor nucleophile.
LDA is hindered by sterics, meaning it cannot attack electrophiles as a nucleophile (very poor nucleophile). It will only act to deprorontate things.
For this reason we can use LDA to fully create the enolate ion from a carbonyl.
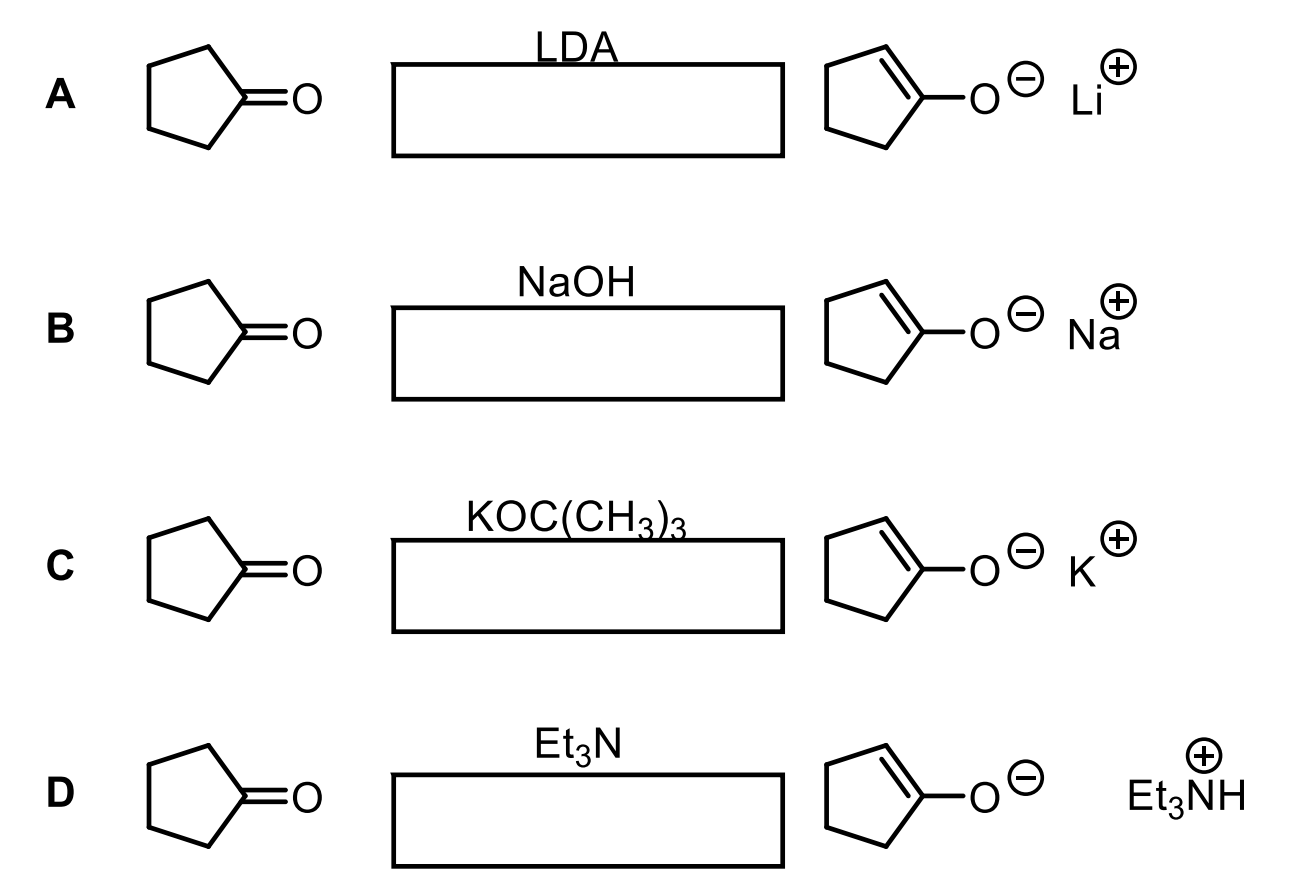
Explain each of these and where the arrows should go.
Equilibrium. Remember, we will favor the acid that is the most stable. So whichever one has the highest pKa will be made the most.
LDA + Ketone. Ketone has a pKa of 20. The conjugate acid of LDA has a pKa of 36. For this reason, we will favor deprotonating the ketone (to an enolate) and forming the very stable protonated LDA (36).
Ketone (20) -OH (conj. acid 14). We will favor having the ketone but could make some of the deprotonated ketone to enolate. It is much more stable to keep ketone as the acid since it is more stable than water.
Ketone (20) + OC(CH3)3 (17). We will favor the ketone becuase 20 is a more stable acid than 17, but it is possible to make some enolate and have a protonated OC(CH3)3, more than with -OH.
Ketone (20) and EtN (about 9.4). The EtN protonated makes an acid with a pKa of 9.4 which is very acidic compared to the ketone so we will heavily favor keeping the ketone as the acid and the EtN as the base so almost no enolate at all. Don’t rely on enolate forming.
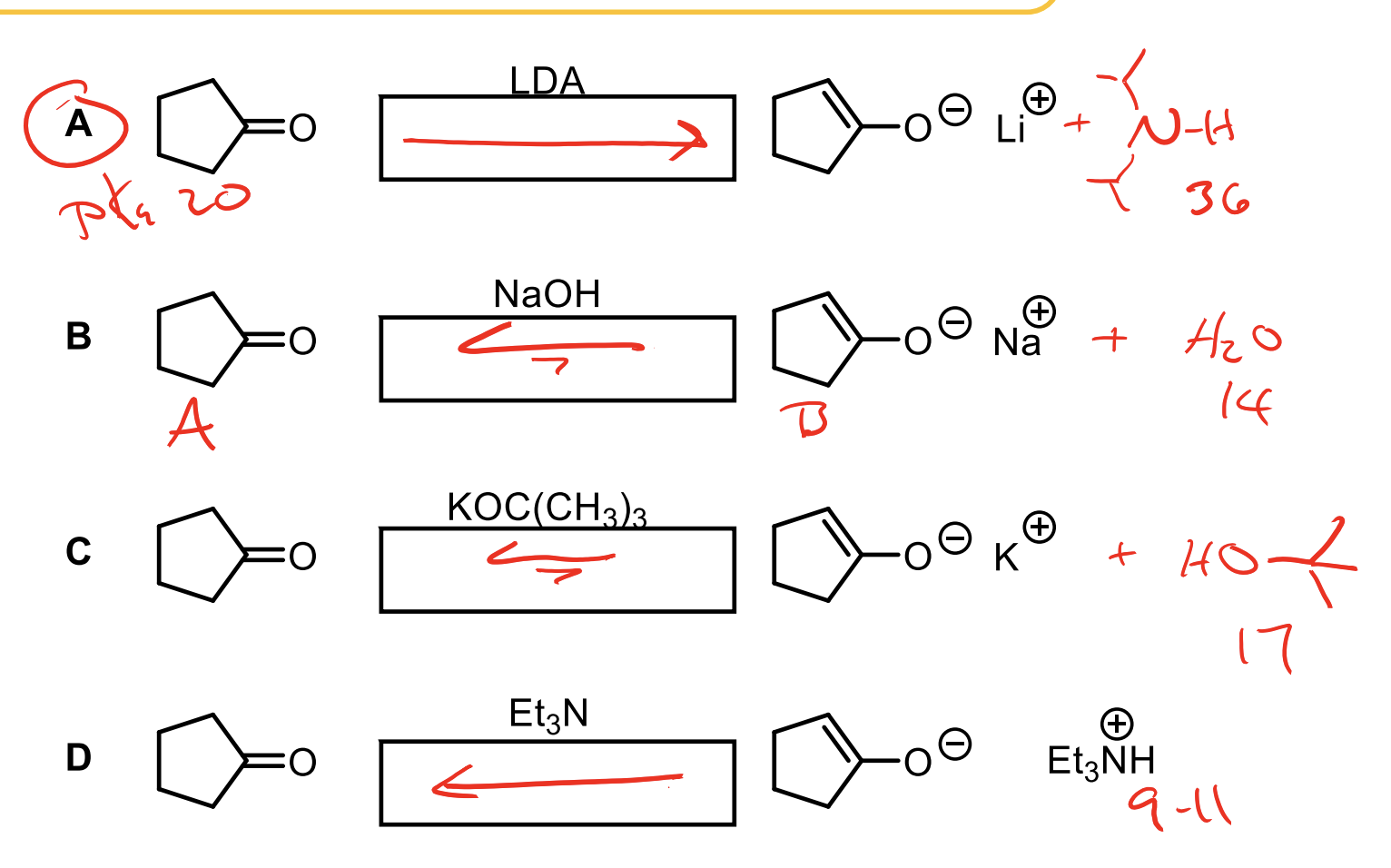
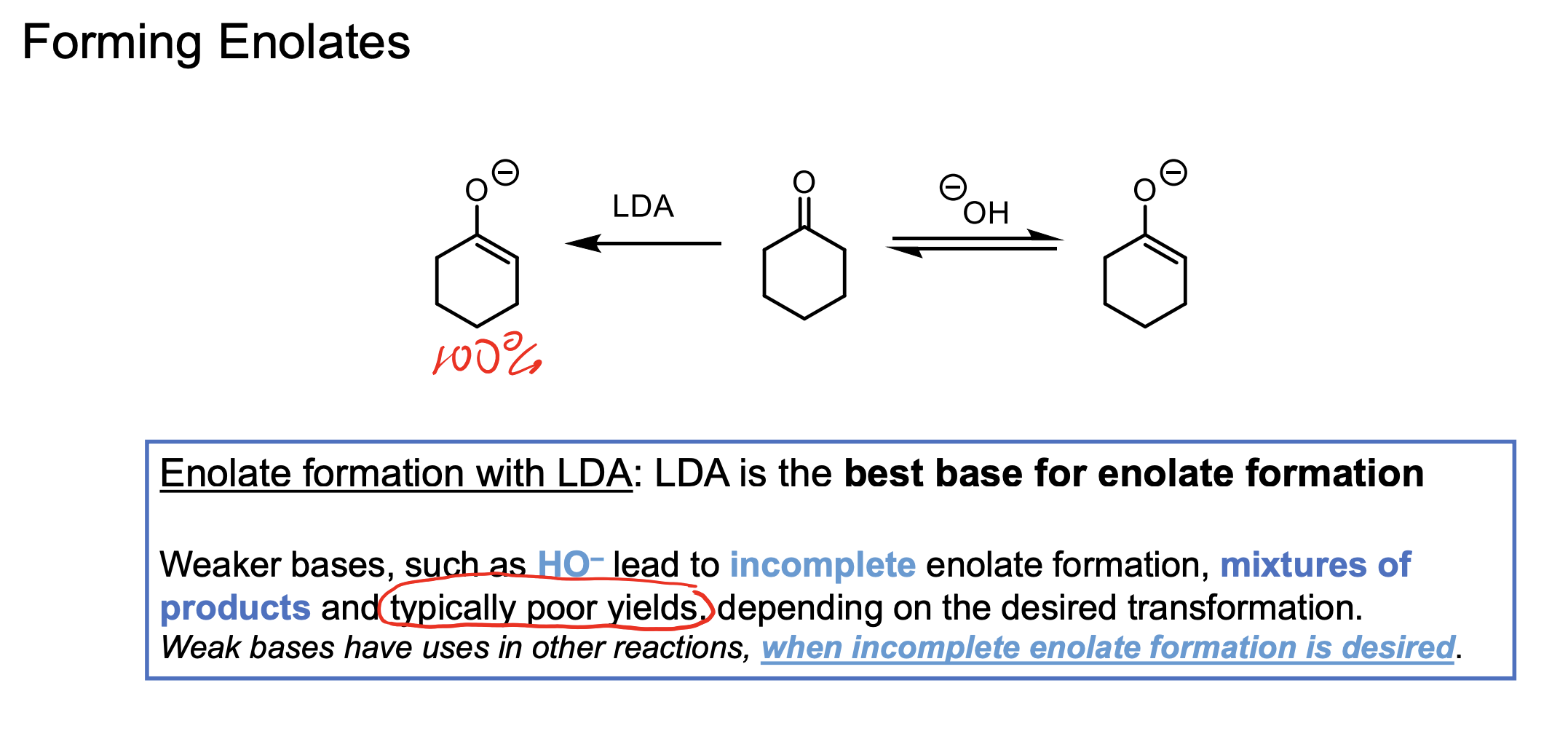
What base can be used to make 100% enolate from a ketone. What about a mixture of enolate and ketone?
LDA will make 100% enolate.
Weaker bases (conj. acid between 14-19) will make enolate at progressively less amounts the lower the conj. acid pKa.
The higher the conjugate acid’s pKa, the stronger the base — and the more completely it will deprotonate the carbonyl to form an enolate.
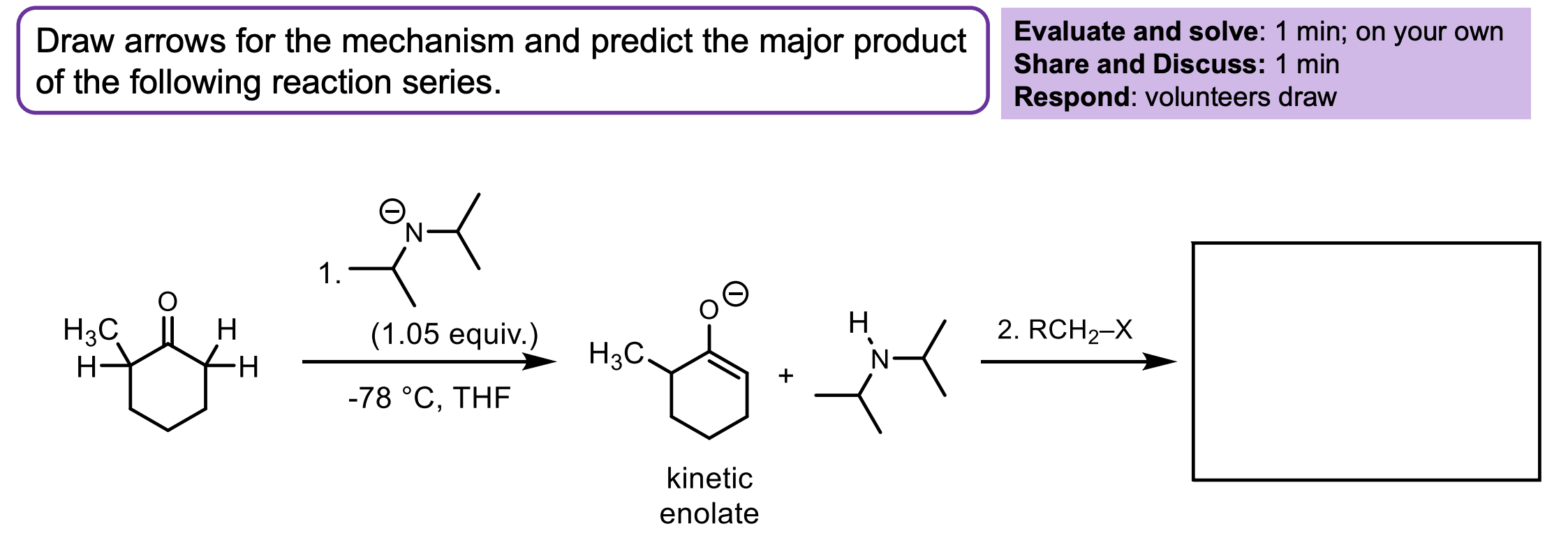
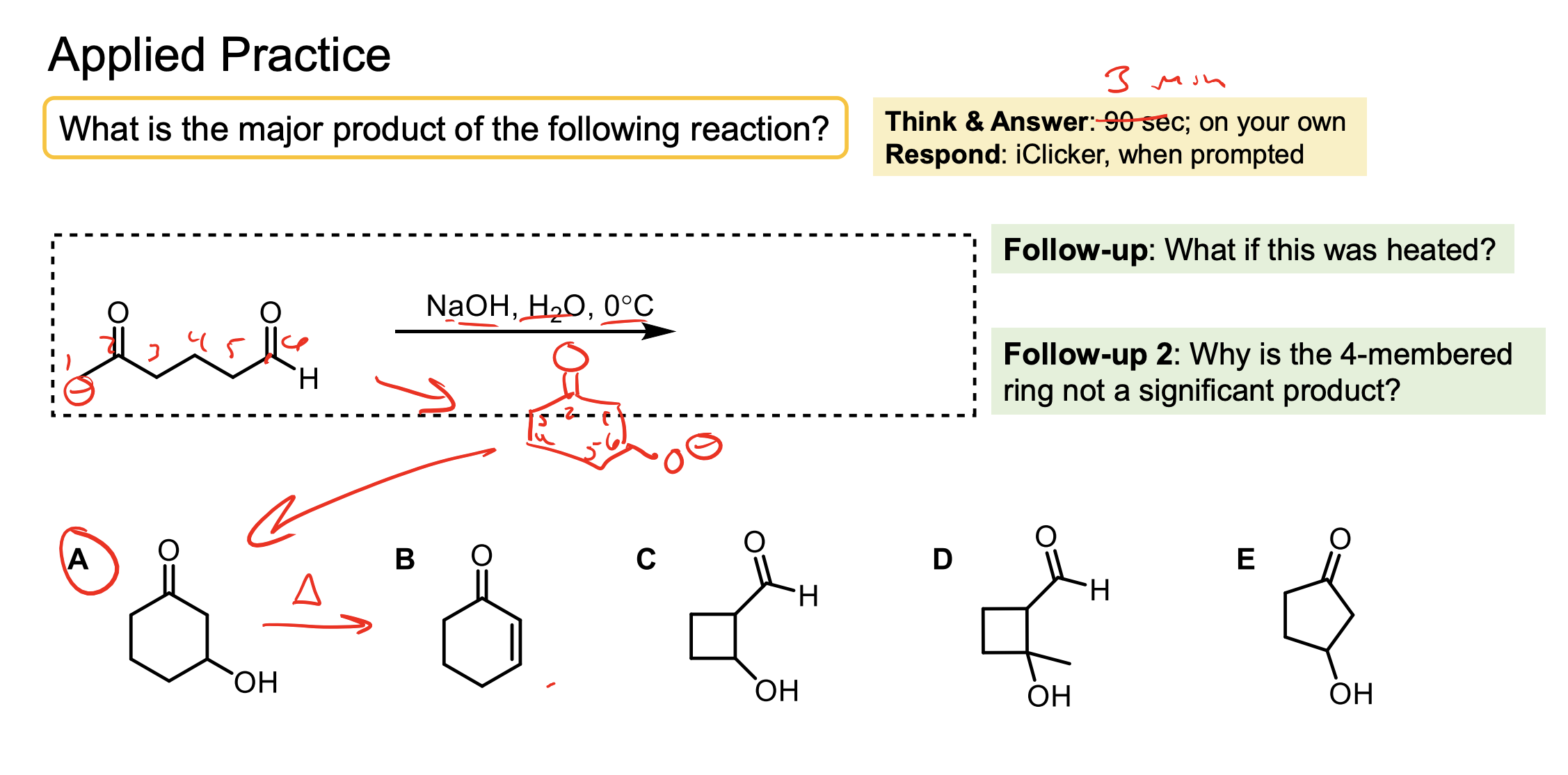
Draw the product and explain what the temperature means and any other conditions…
We have a ketone. The temperature is very cold so only the kinetic product will form.
Remember, the double bond is happier with more methyl groups branching off, but it is harder to deprotonate that hydrogen on the left because of sterics and becuase it is less acidic. So at -78° one of the hydrogens on the right gets deprotonated. We form the enolate which then attacks the alkyl halide to form the substituted product on the right.
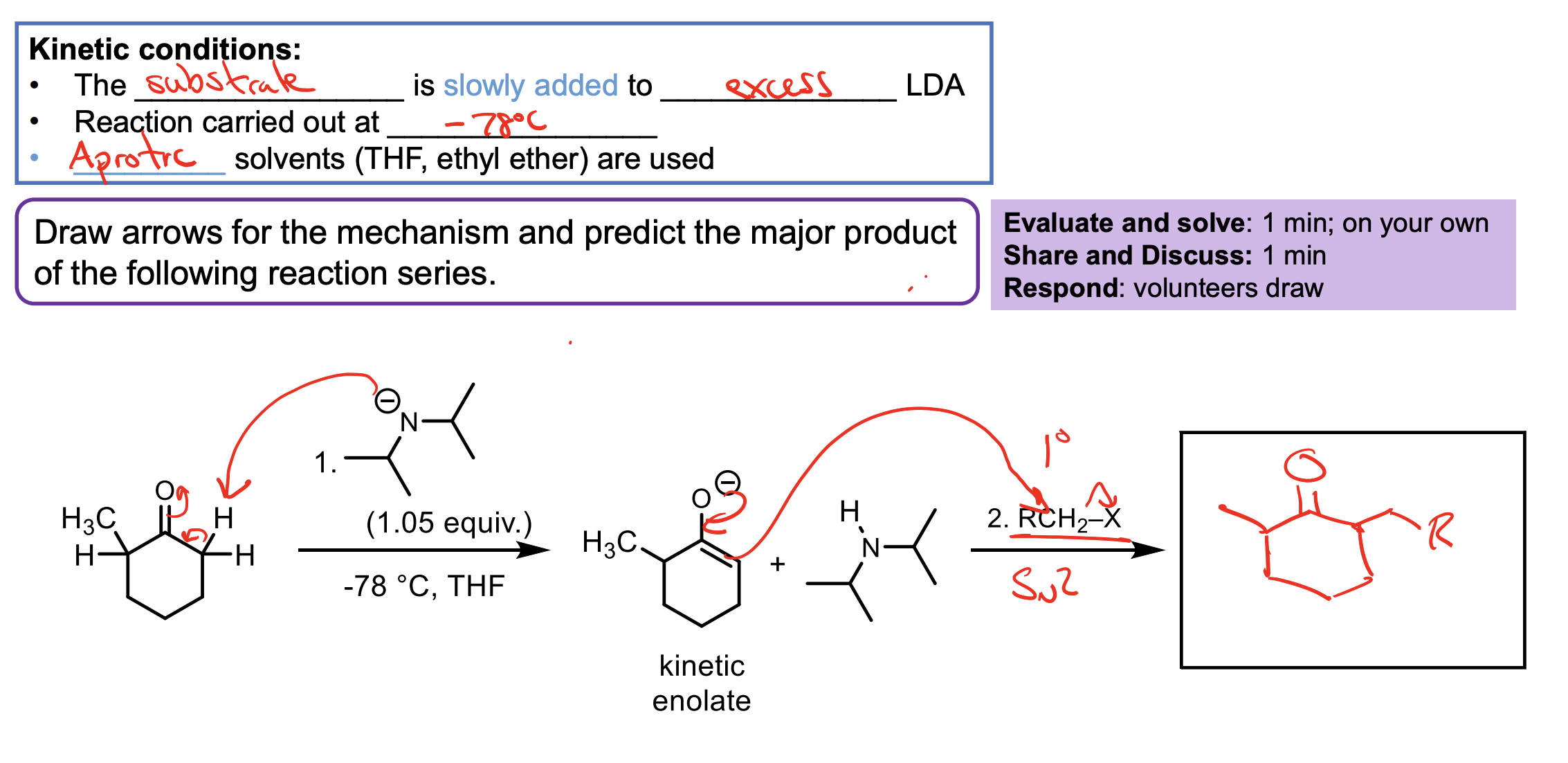
We have to have excess LDA and we have to use THF or ethyl ether.
The LDA is in excess so we get all the ketone to enolate ion. We HAVE to add the ketone slowly so it doesn’t react with itself when it turns to an enolate.
We need the aprotic solvent like THF or ehtyl ether to avoid protonating any of our LDA with our solvent and making it not useful for our reaction (if we didn’t have an aprotic solvent some of the LDA wouldn’t protonate the ketone and the ketone could react with itself as an enolate).
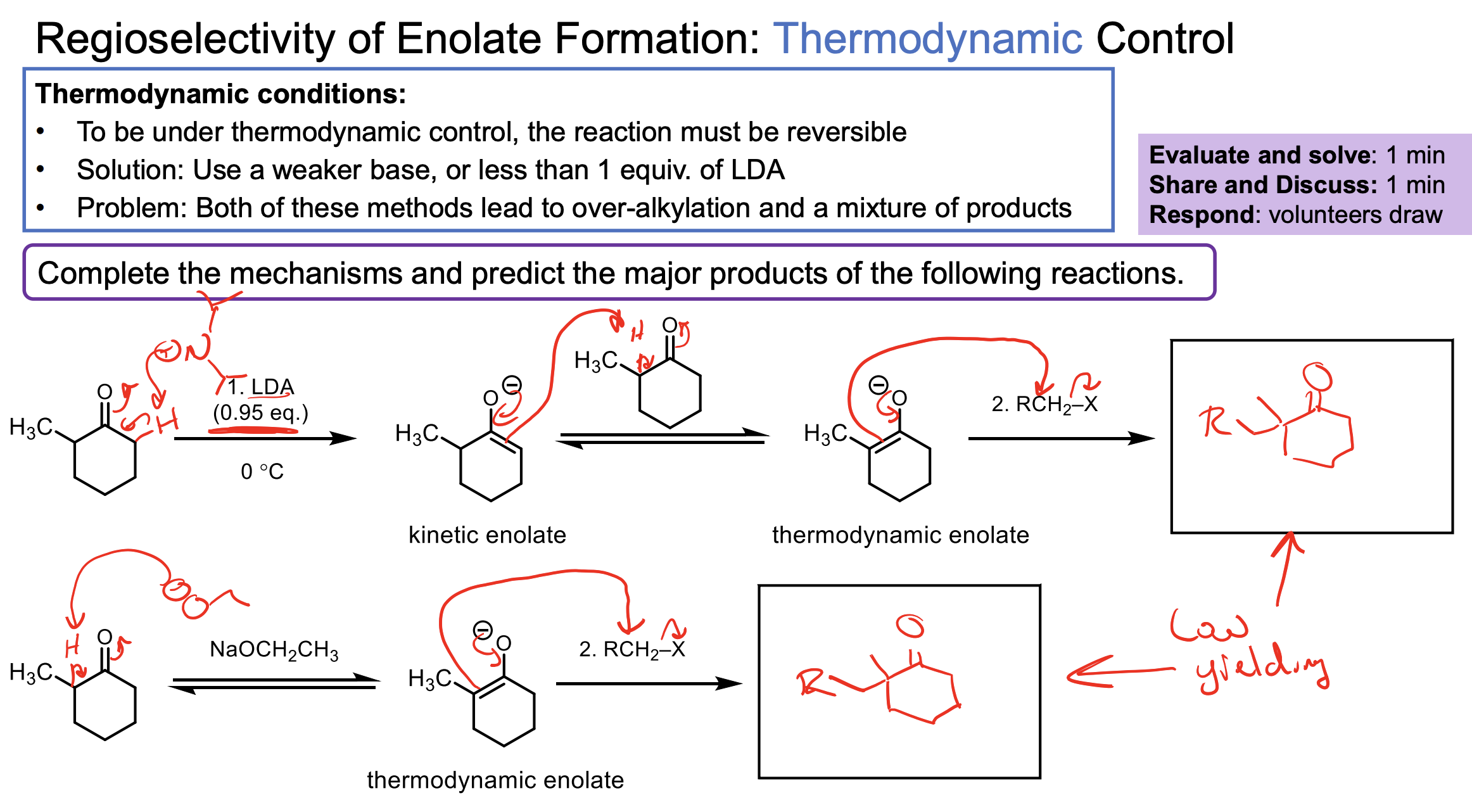
Why do we not see overalkylation in the formation of the kinetic enolate reacting with an alkyl halide?
Why do we see overalkylation int he formation of the thermodynamic enolate when reacting with an alkyl halide?
Once you add the alkyl halide to the kinetic product you know all of the LDA was used up and you have all enolate. So all of this enolate will react with the alkyl halide and there is no more LDA to reconvert it back to a new enolate. It is too cold to do the interconversion.
Once we add our alkyl halide we will have a mix of ketone, enolate (both types) and the alkyl halide. Some enolate will react with the alkyl halide and form a ketone product. Becuase of the temperature, the enolates that are kinetic could technically deprotontate the newly formed ketone and make that enolate product which could then react again with the alkyl halide. Not all will happen at once. This DOESN’T occur with our kinetic process becuase in that process its so darn cold this interconversion isn’t possible.
The important thing about this slide is that the interconversion of kinetic enolate to thermodynamic is possible here but not in the cold.
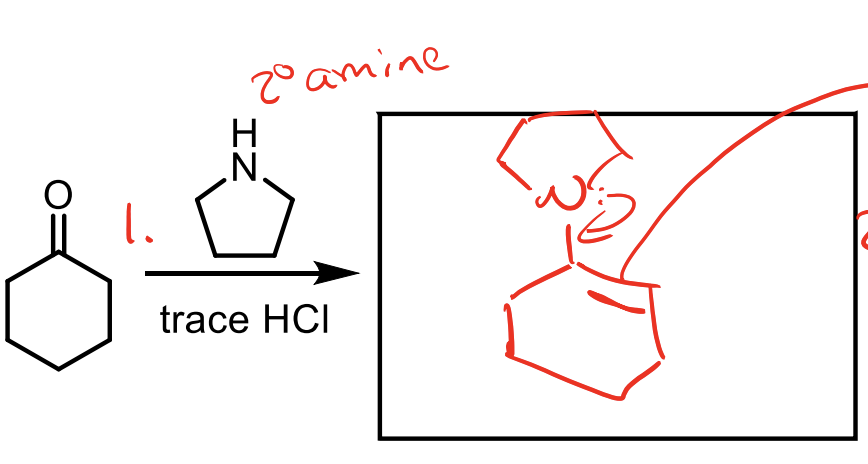
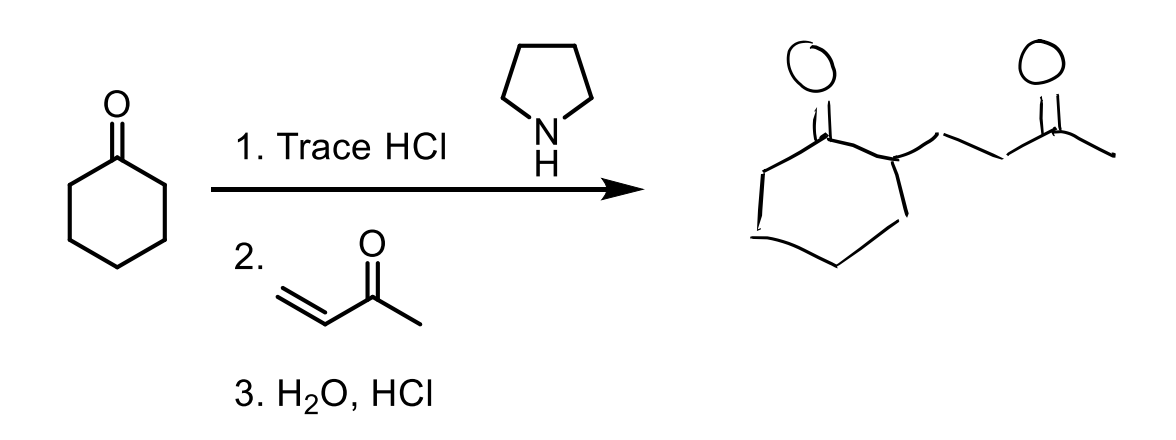
how do we form an enamine when we have a ketone?
We give the ketone an amine with two R groups and an H. If we gave it 1 R group or no R groups, it would just make an imine.
This is important because an enamine functions very similar to an enolate.
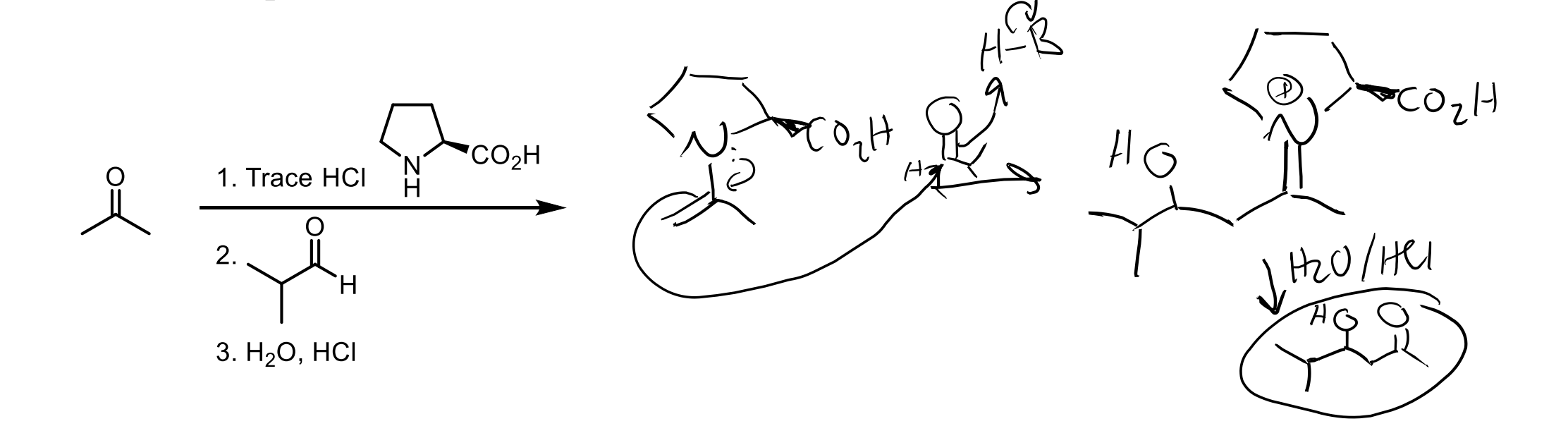
Remember, you need the amine and trace HCl to protonate the O twice after the amine attacks so it can push it off. (it is in acid so the O gets protonated first then amine attacks).
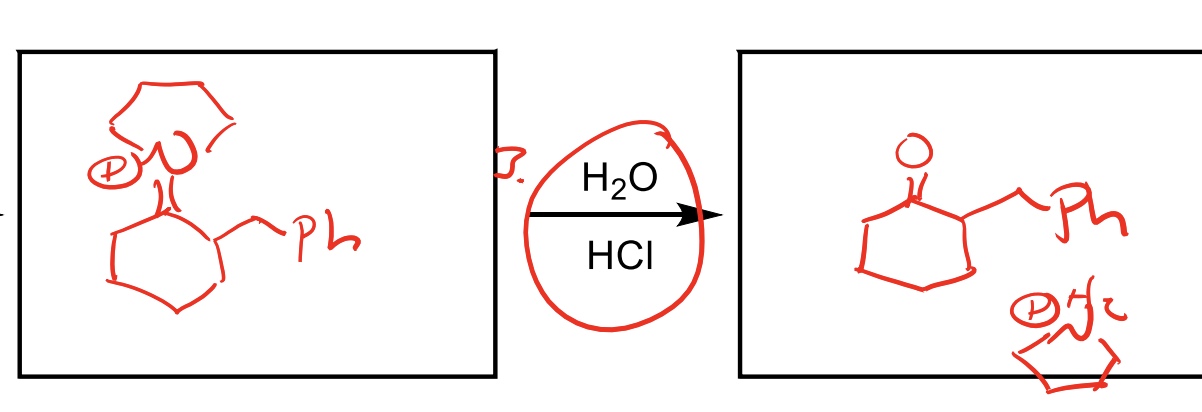
How do you reverse an enamine?
You can reverse an enamine back to a ketone with H2O and HCl.
In this picture you are basically showing the middle of the hydrolizing mechanism so don’t get confused!
Remember the mechanisms last step is to deprotonate an alpha hydrogen, so the first step would be to protonate the alpha position by pushing the electrons down from the enamine Nitrogen, then attacking with that H2O the N+…you get the gist.
Generally the (more/less) substituted enamine is formed due to ____
Generally the less substituted enamine is formed due to sterics.
What can enamines react with as carbon nucleophiles?
Enamines can react with 1° halides and acyl chlorides to add carbons.
You then have to follow that up by using H2O and HCl to hydrolize the imminium ion formed back to a ketone.
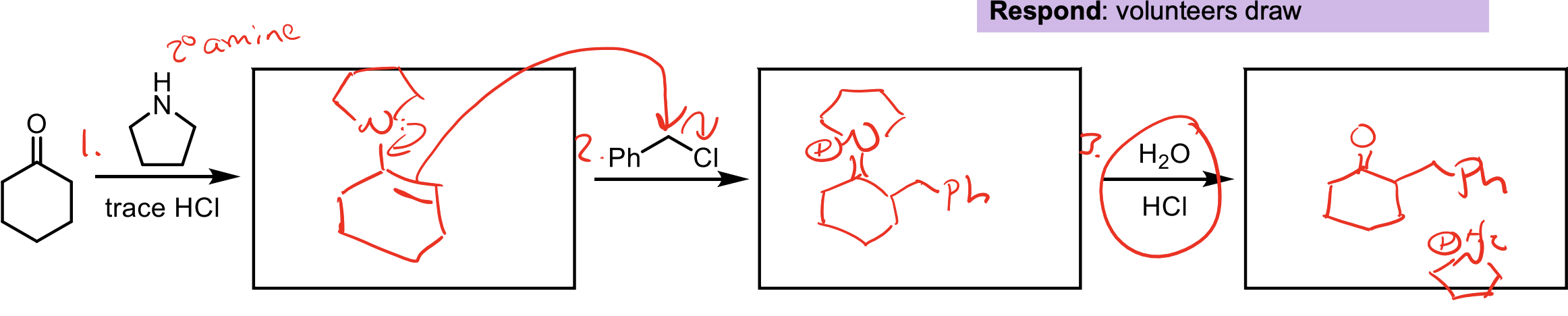
Enamines can be used as nucleophiles. You can add an enamine to a ketone by using an amine with two R groups (like the one shown) and HCl.
Then you react in a second step with the 1° alkyl halide to add carbons.
Then you can reduce it back to a ketone with H2O and HCl
Three Steps to add carbons to a ketone.
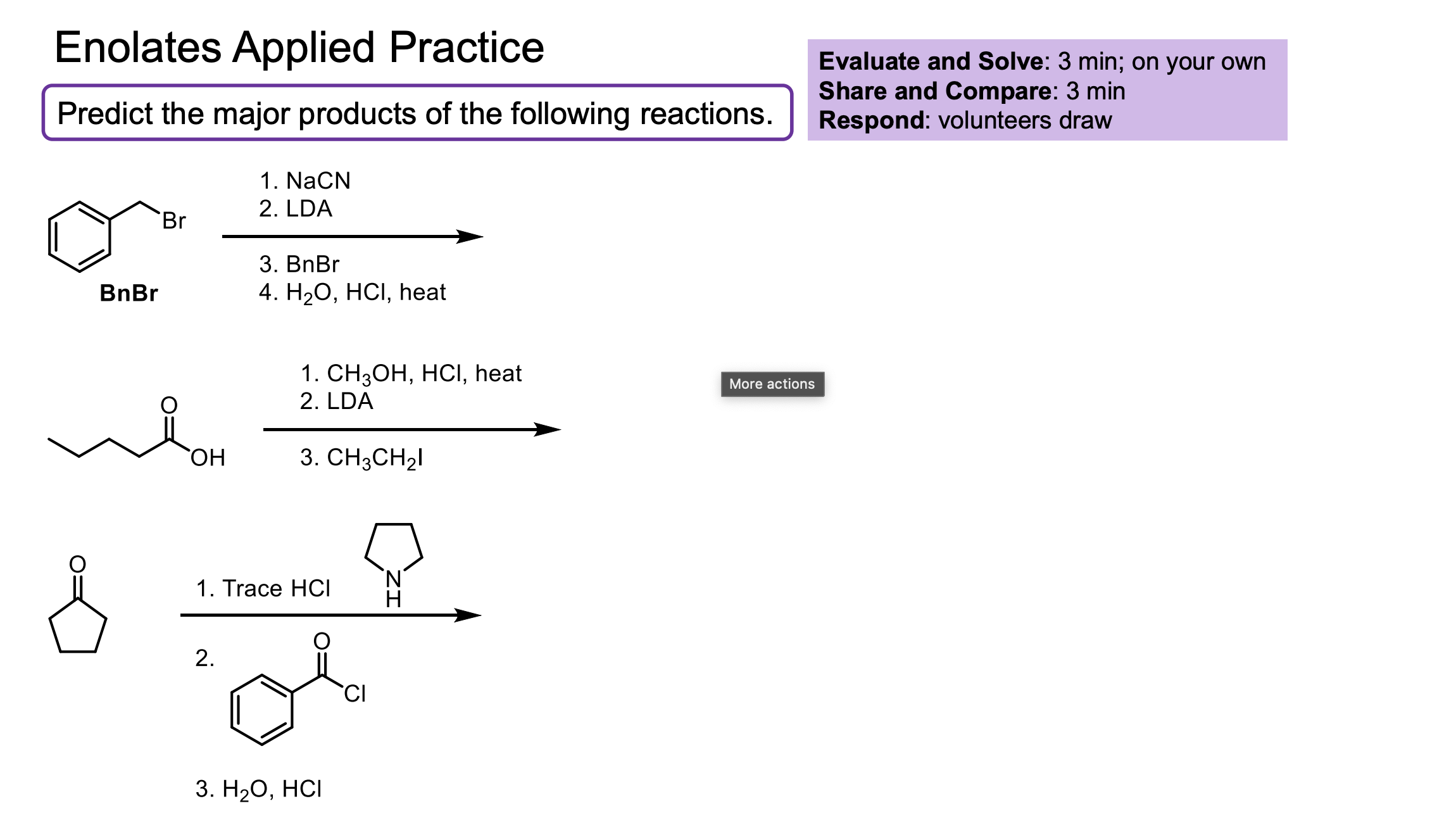
With these enolate reactions make sure to not forget about asymmetric centers and enantiomers/diastereomers!
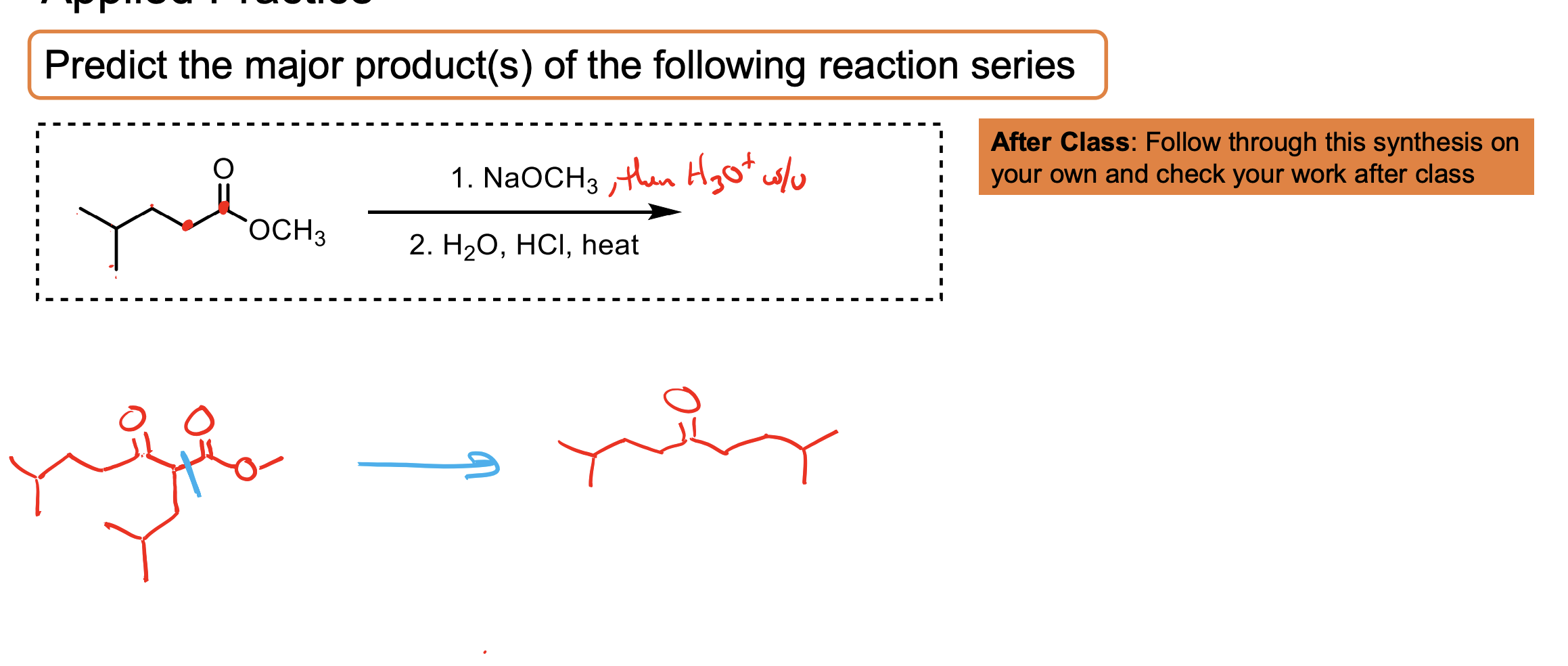
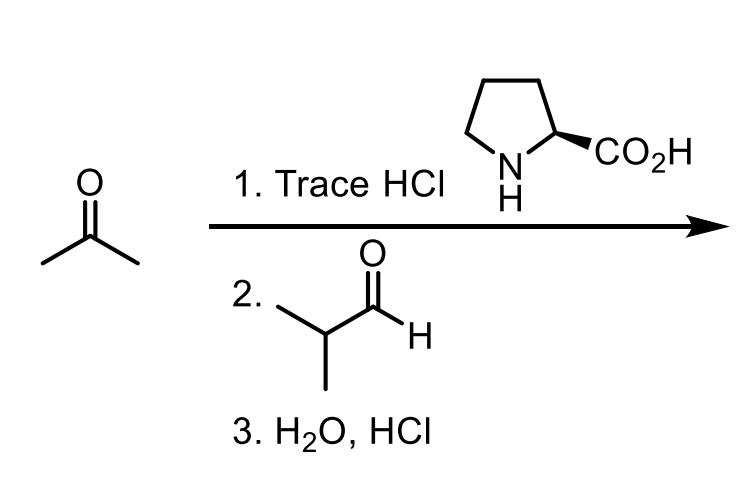
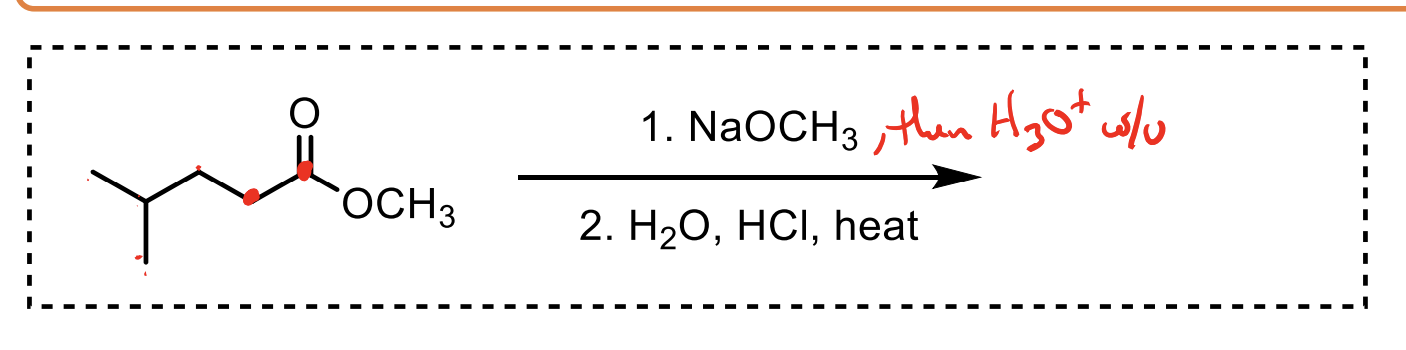
How do you convert a carboxylic acid to an ester? Why might you do this in enolate formation?
Convert a carboxylic acid to an ester by adding the alcohol, HCl, and heat ∆.
You would do this because the carboxylic acid hydrogen (OH) is more acidic than an alpha hydrogen, meaning if you used LDA for example, it would just make a whole heap of carboxylate.
How do you convert an ester to another ester?
Trans esterification. This uses the alcohol you want, HCl, and heat.
The LA said that for like the other one if you don’t use the alcohol that matches it could do trans esterification a little bit (even though conditions aren’t exact) which would fuck up the reaction. These conditions are the ones to make it ALL the transesterified product and all that jazz.
I am a nucleophile. Where will I attack explain the rules.
If I am a H- (LiAlH4 or NaBH4) I will attack the ketone from the 1,4 and then attack again.
If I am a grignard I will attack the 1,2 no matter what.
If I am literally anyone else, I will attack the 1,4.
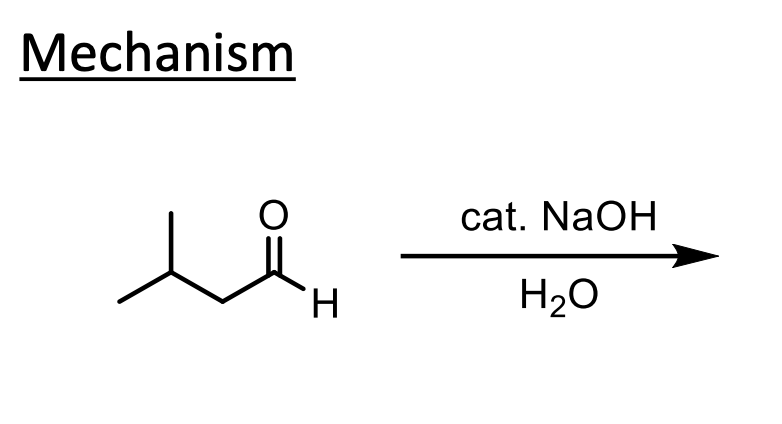
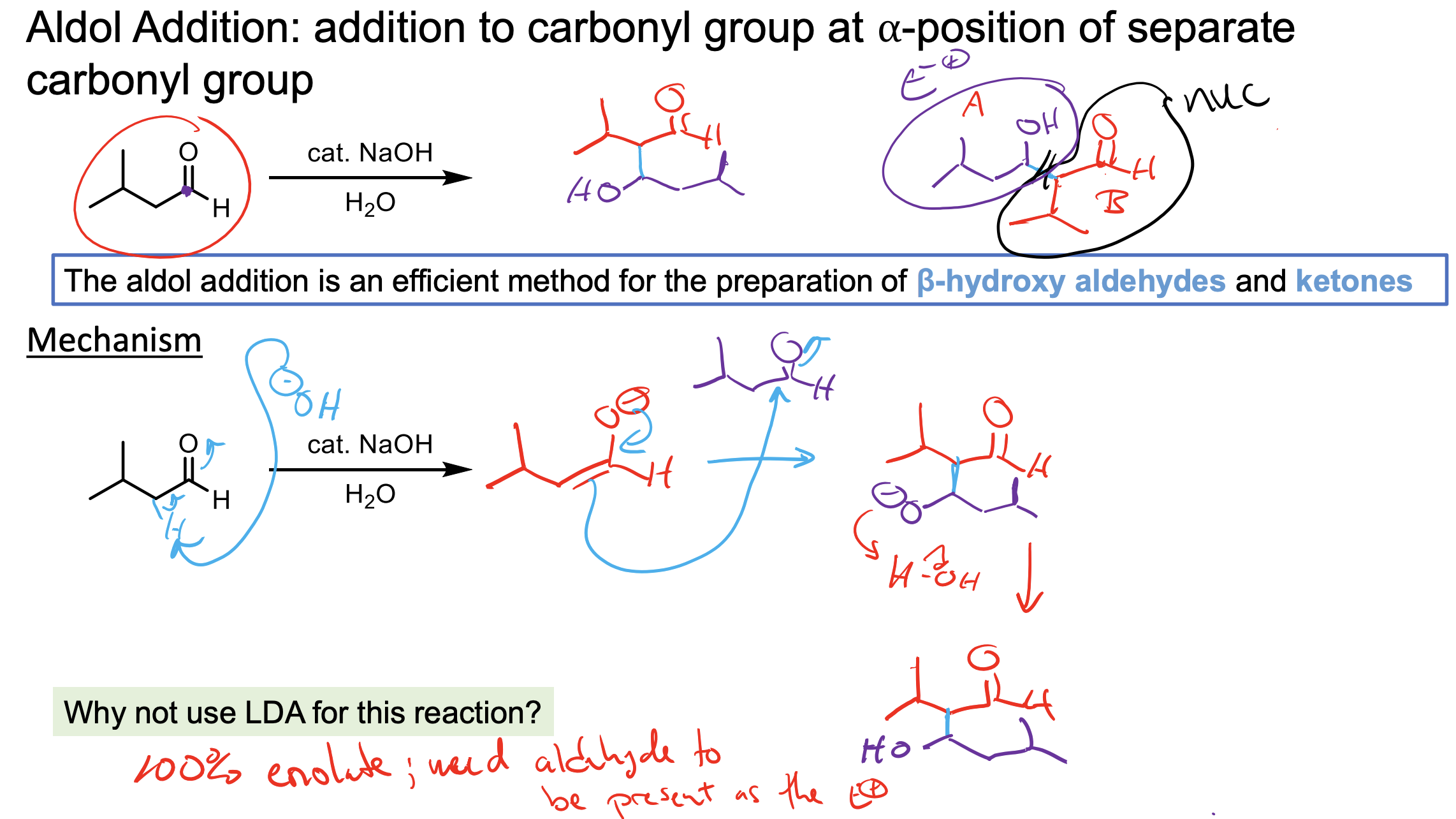
What does this do? Why is the OH catalytic? Why not just use LDA?
We form a beta-hydroxy-aldehyde.
We form some enolate which will react with itself to push up to form an O- with nothing to kick off, so water will be deprotonated to form the product.
The NaOH is catalytic because it is used to deprotonate (forms water) then that water is what is used to protonate the O- which reforms the -OH.
We do NOT want to use LDA in this reaction becuase we would just make all enolate so there would be no ketone left to react with.
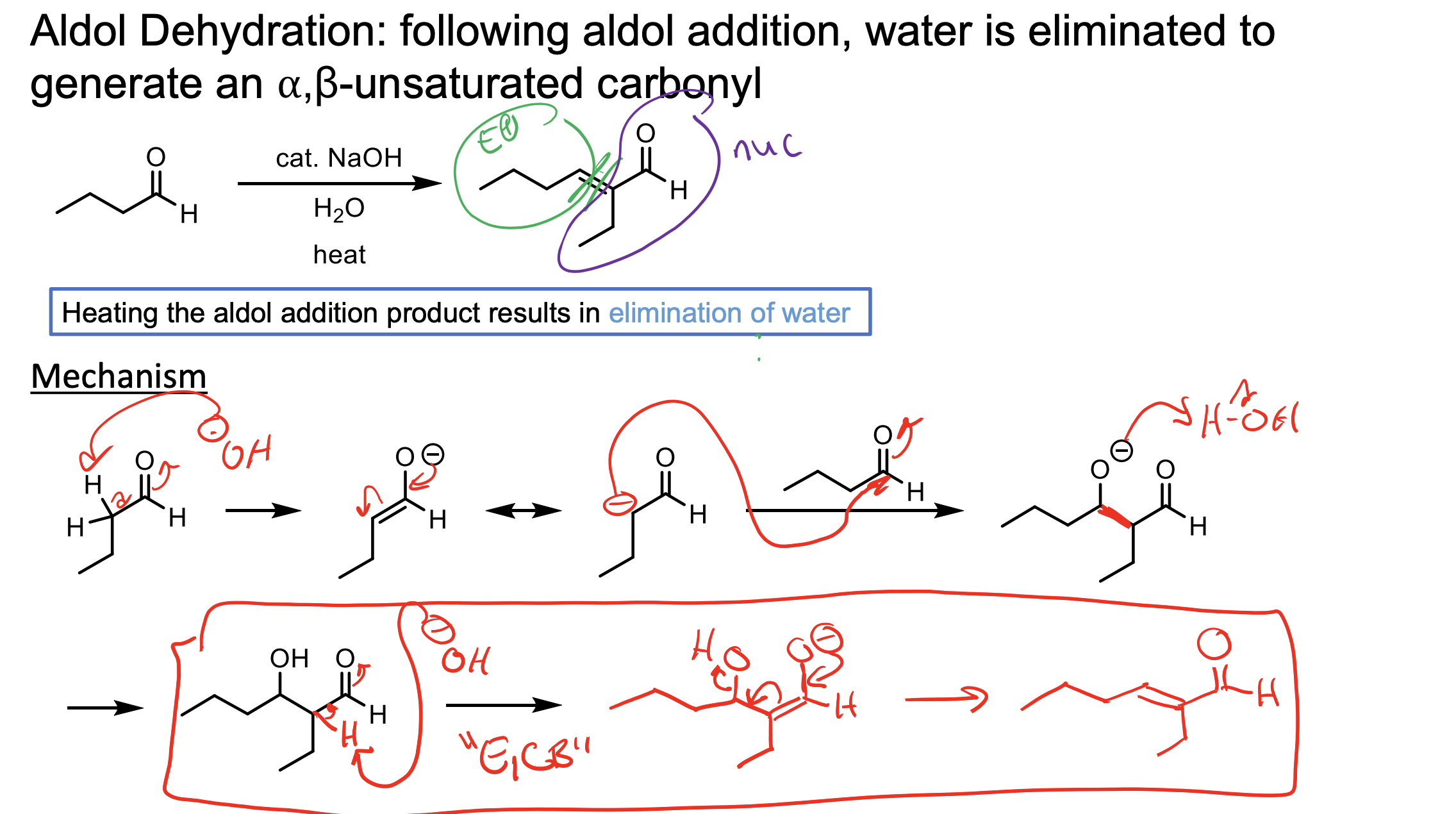
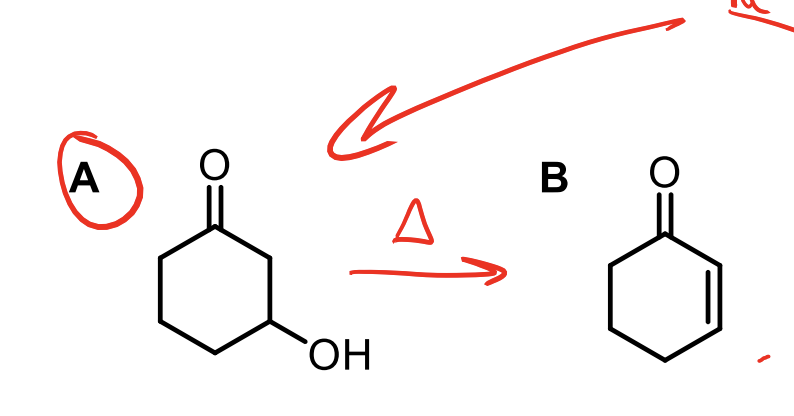
What does heating the aldol addition product result in?
The elimination of water.
This is the E1CB reaction. You HAVE to make the enolate intermediate seen in the second step in red. This then pushes down to push off the OH.
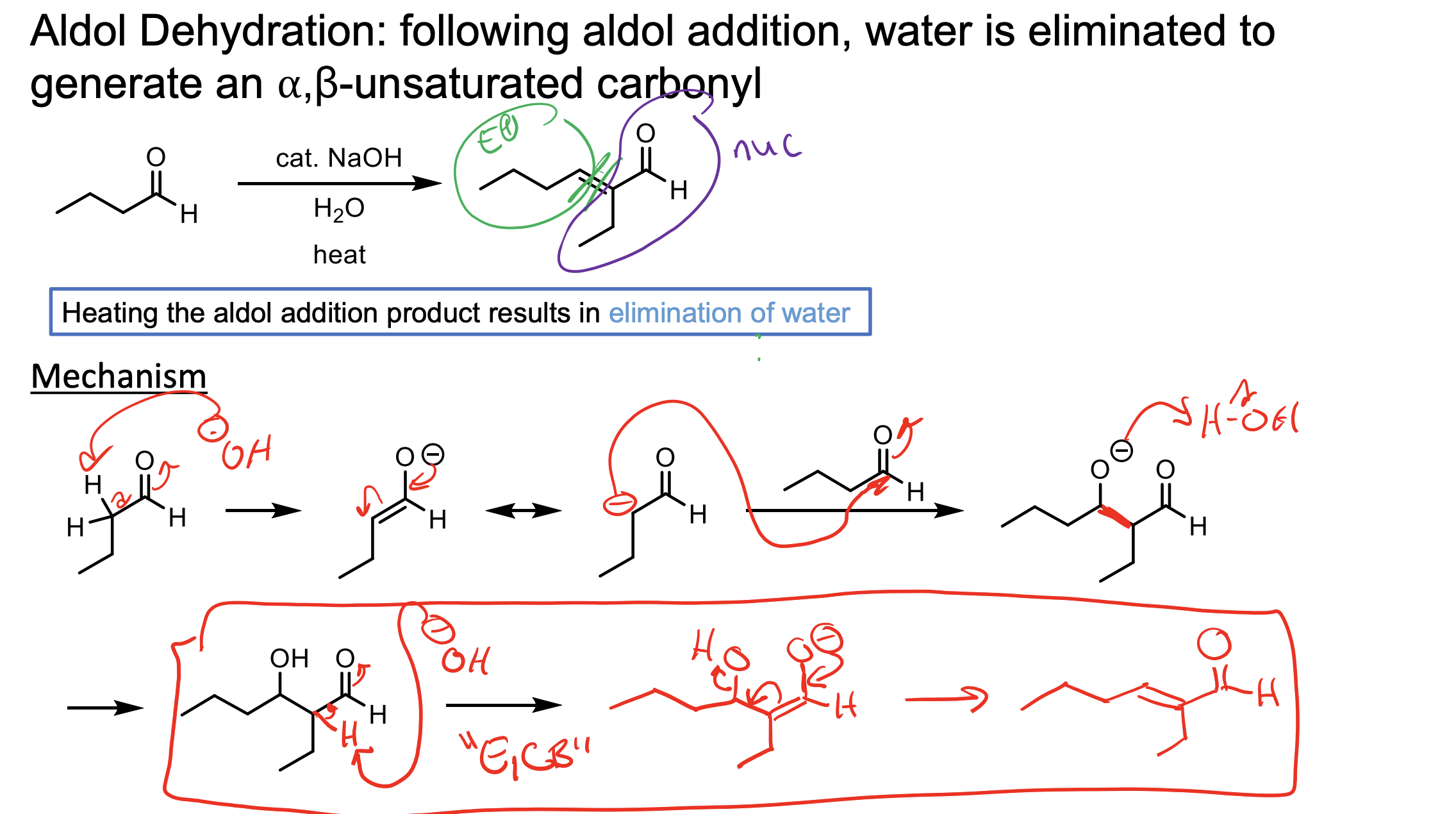
Remember, heating the aldol product results in the elimination of water through that weird enolate intermediate (must be formed).
You WILL form the aldol product, but the heat will cause the -OH you created to deprotonate the alpha hydrogen again and make another enolate that will then push off the OH.
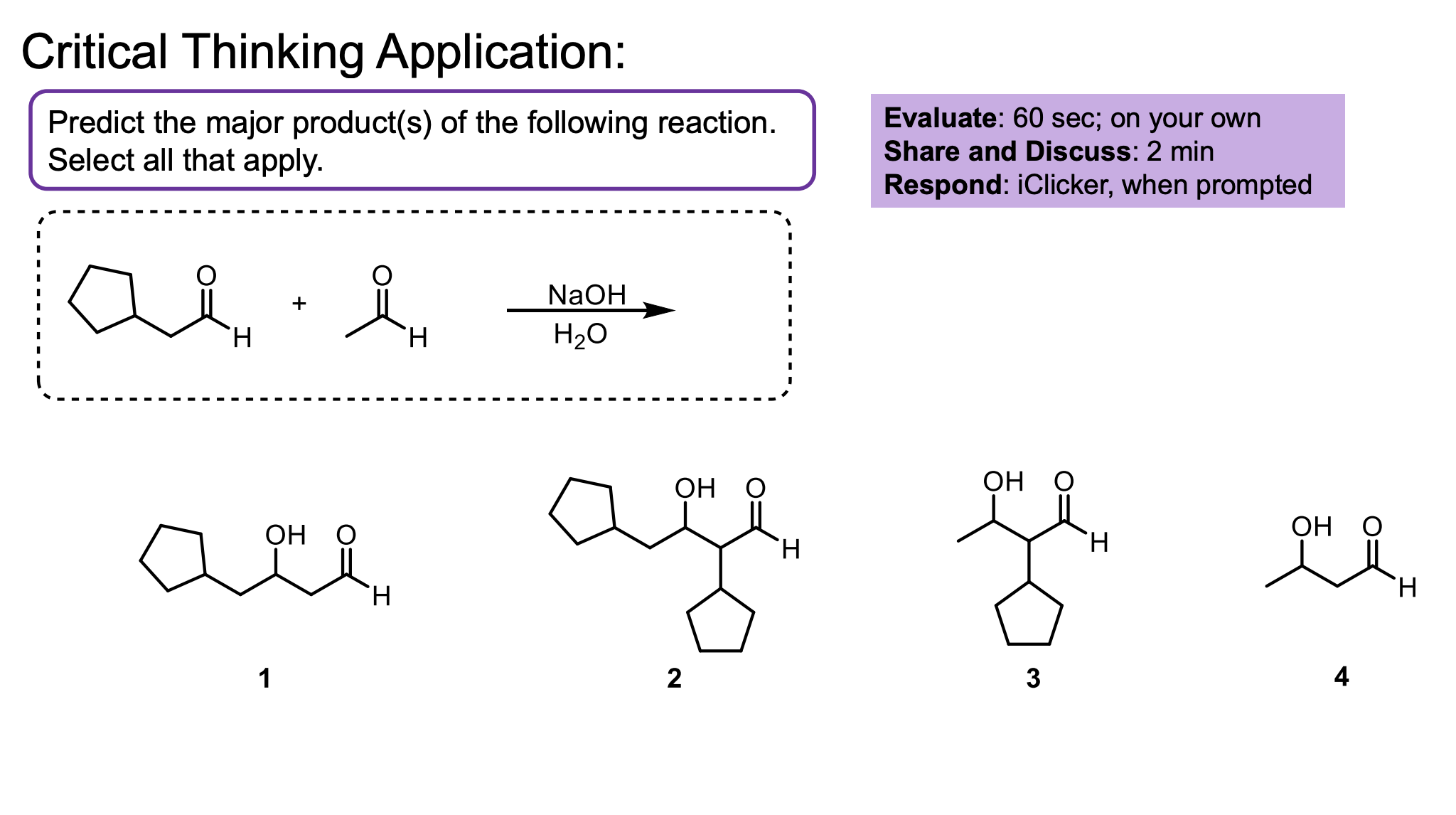
Both could be the nucleophile (they were converted to the enolate).
Both could be the electrophile (they were kept as the ketone/aldehyde).
That means both could bond with themselves or with each other, making 4 products.
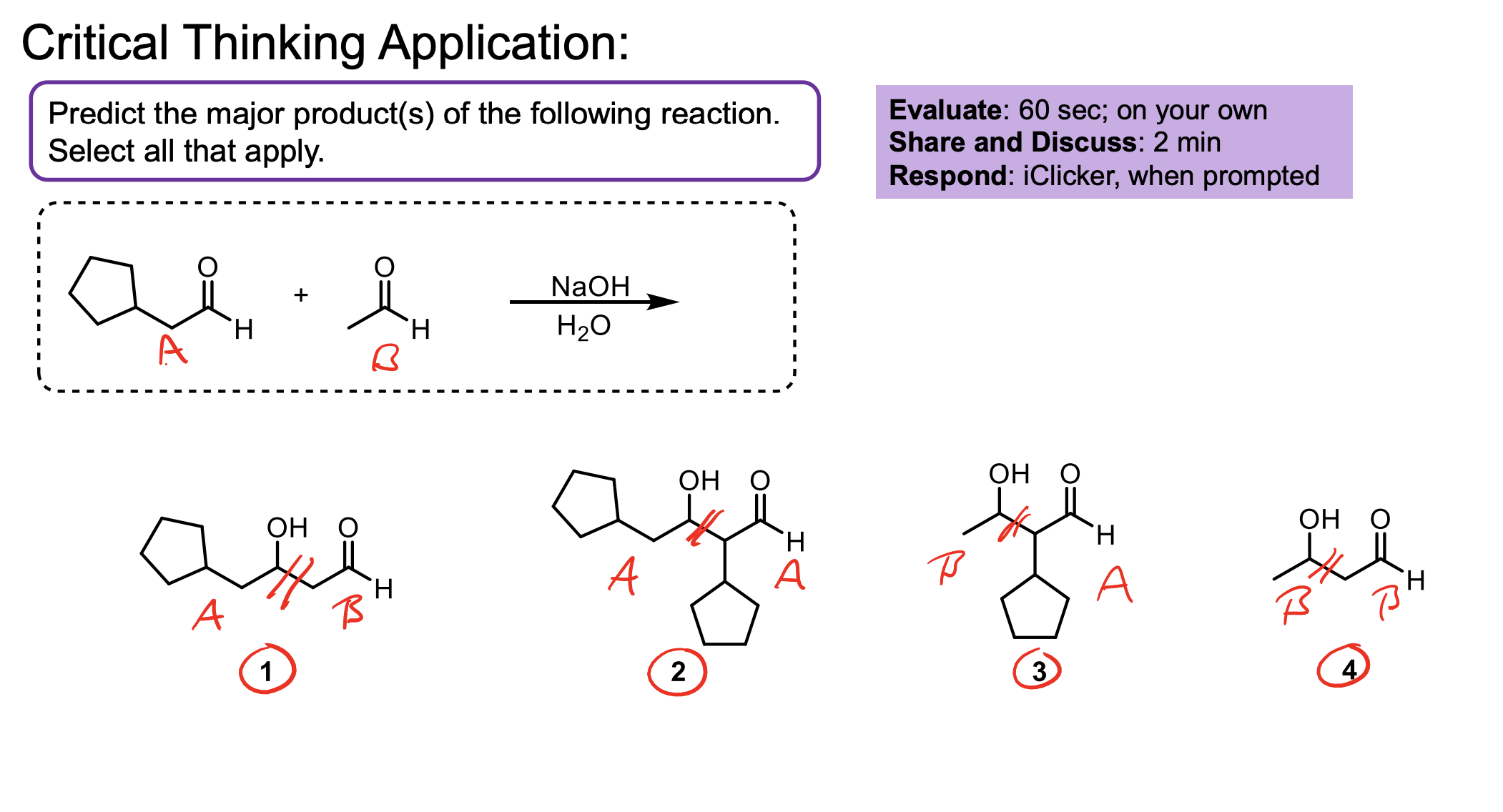
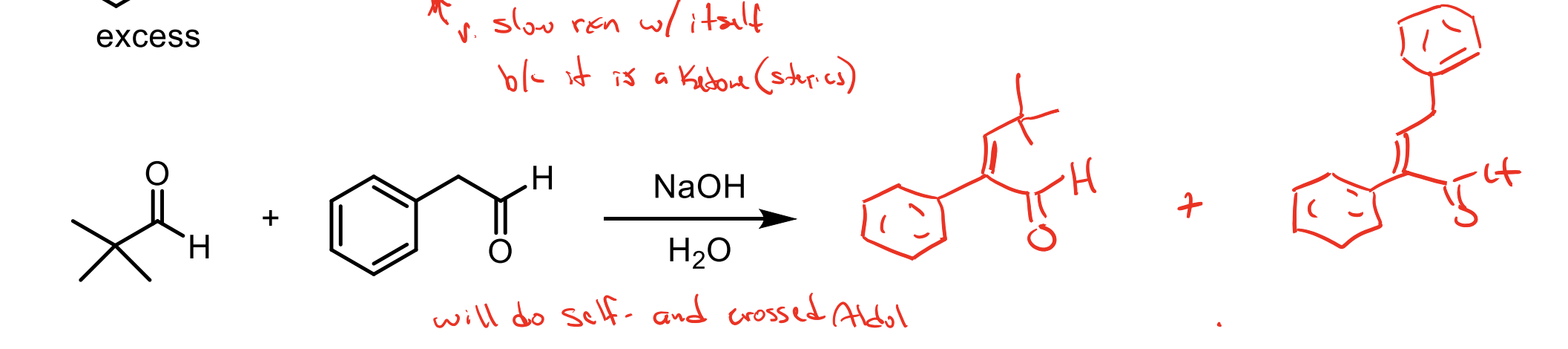
What are the two methods used to get major products using crossed aldol addition?
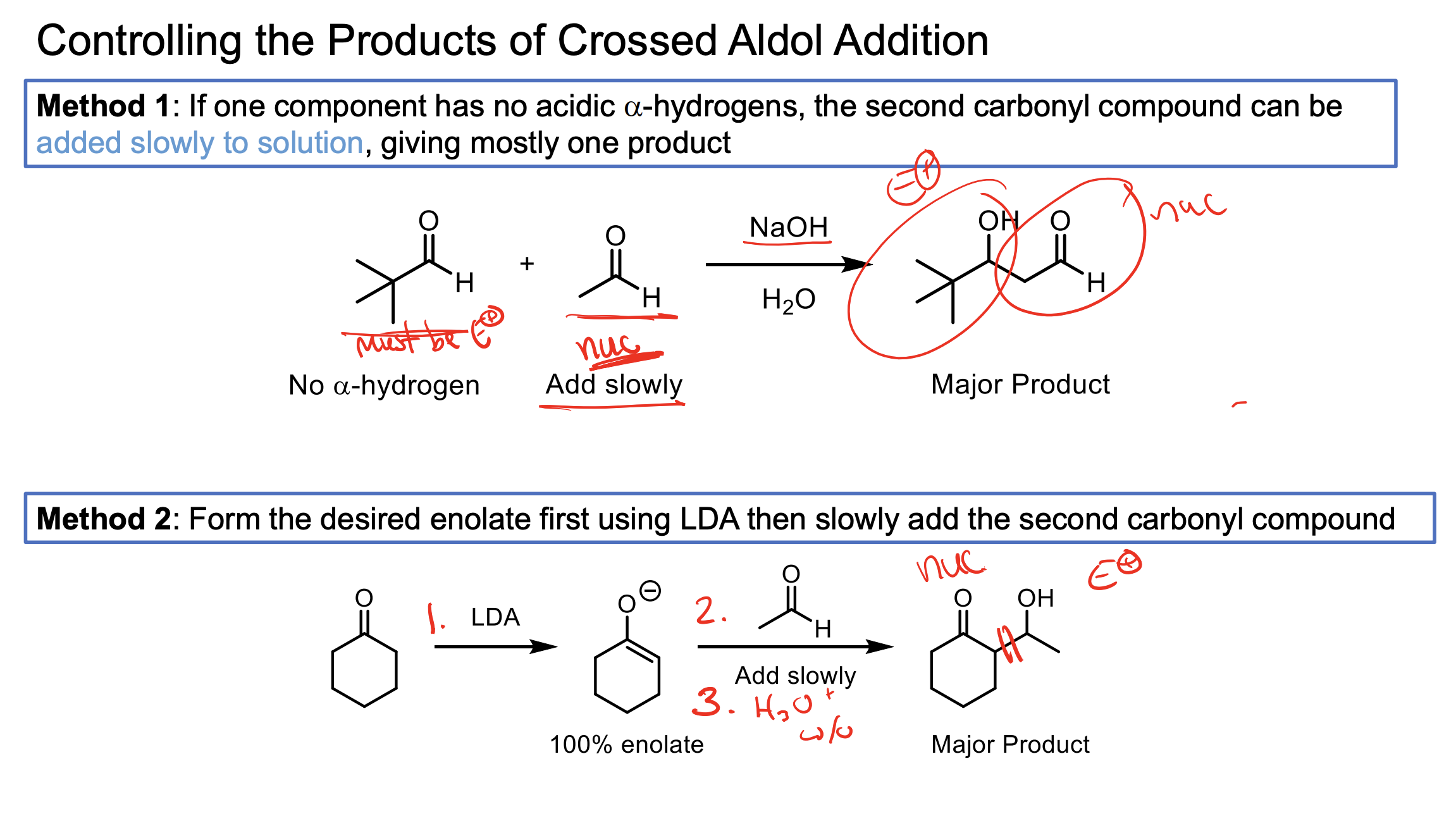
If one of your ketones/aldehydes has no alpha hydrogens and the other does…you can add NaOH and H2O in solution with your non-alpha hydrogen reagent, then add in your alpha hydrogen reagent slowly. When the alpha hydrogen one is converted to an enolate, since we are adding it slowly it will only really react with the abundance of non alpha hydrogen product.
If both have alpha hydrogens, we can use LDA to convert one to its enolate form entirely then add in the other reagent slowly. This helps ensure the enolate reacts with all the reagent. You also have to add an H3O+ w/u to make the alcohol at the end since you didn’t have H2O like in the other method.
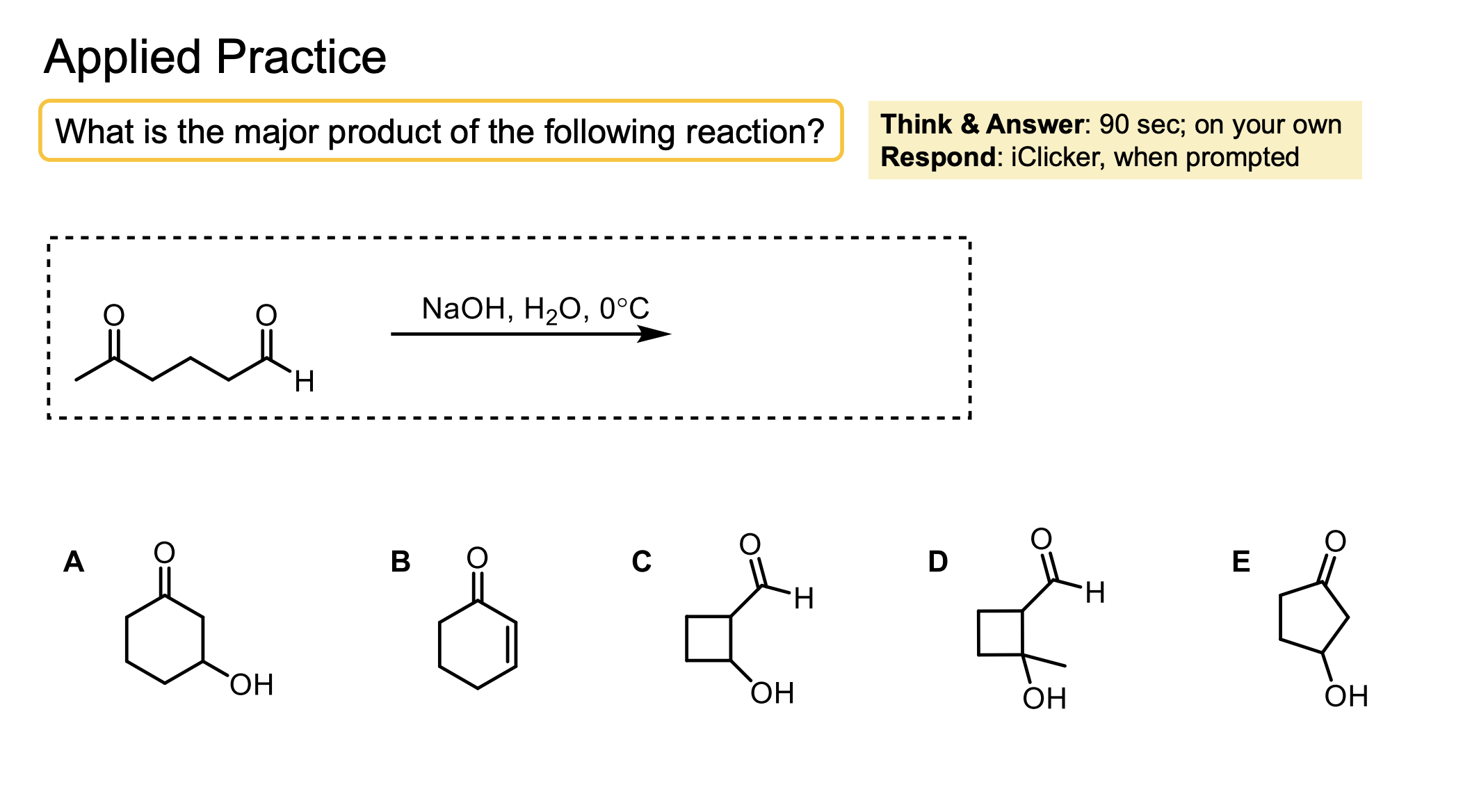
The intramolecular product forms much faster than the intermolecular product so when it is possible it will occur in an aldol reaction.
This can only occur to form 5 and 6 membered rings, so if those aren’t possible it will do intermolecular reactions.
If you were to add heat to the aldol product it will deprotonate another alpha hydrogen then form an enolate again that will then push off the OH to form a beta unsaturated ketone.
Additional Chat GPT Advice
Intramolecular reactions are often faster and favored because:
The reactive sites are already tethered together → high effective concentration.
There’s no need for two separate molecules to collide correctly in solution.
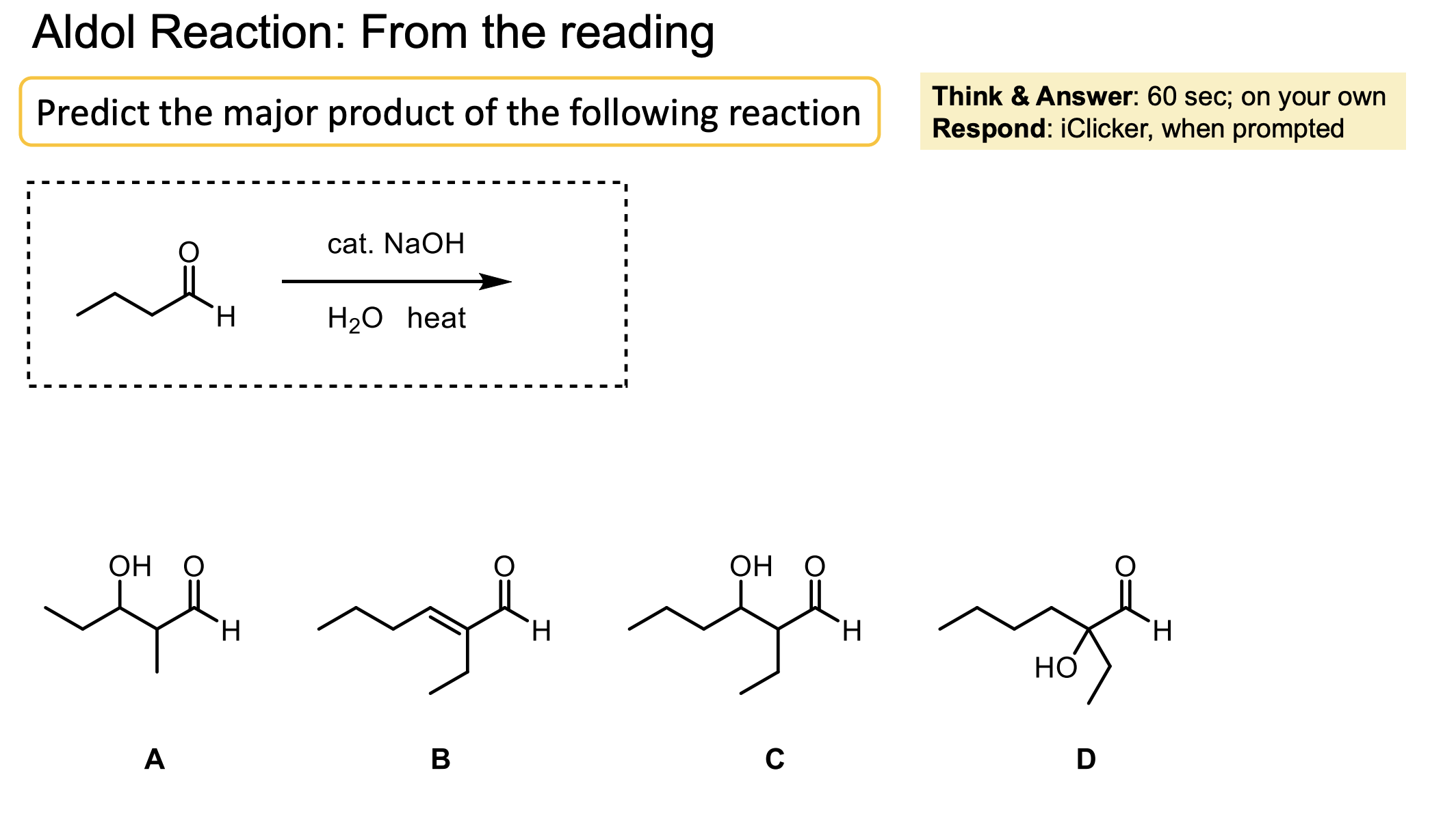
What functional group does heating an aldol reaction form?
A beta unsaturated ketone.
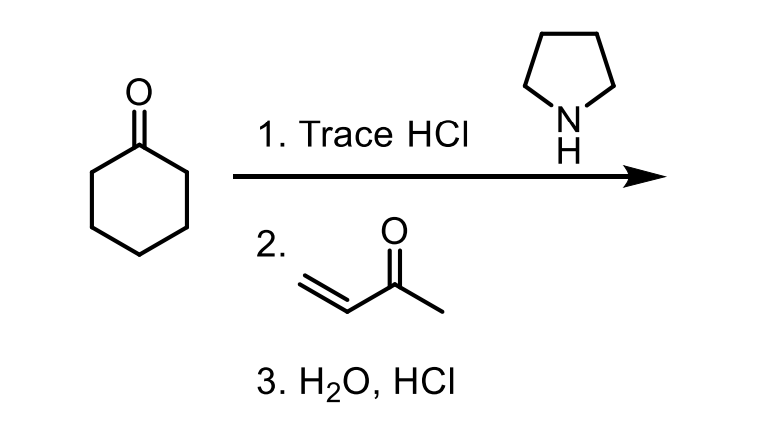
How can I get a beta unsaturated ketone from an aldol? More, how can I do the E1CB reaction?
The E1CB reaction occurs if you HEAT the aldol product OR if the aldol product has a benzene ring which would be conjugated with the beta unsaturated ketone.
For example these ones.
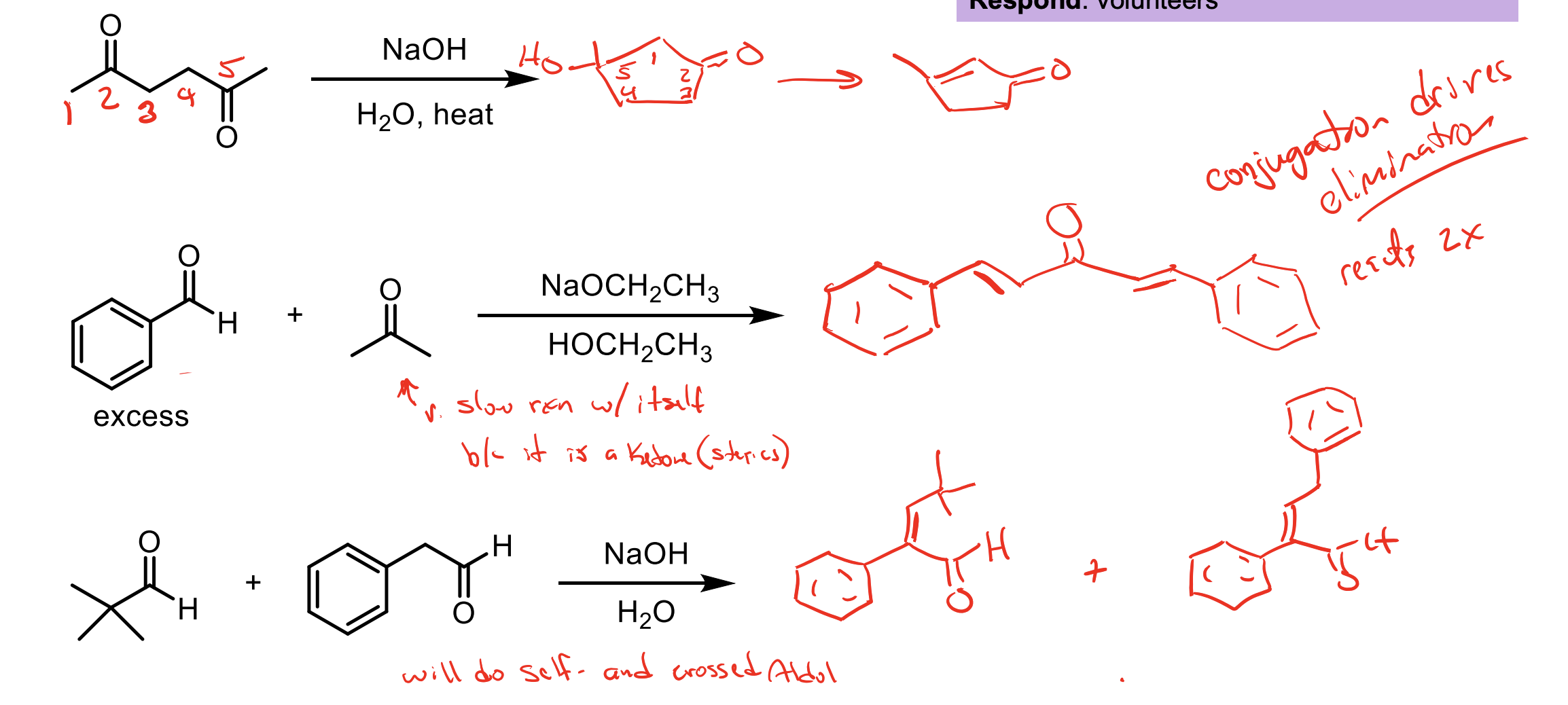
Intramolecular reactions happen faster than intermolecular reactions (if you can form a 5 or 6 membered ring).
Remember, you’ve heated the product so you’ll do the E1CB reaction and push off the OH via the enol intermediate.
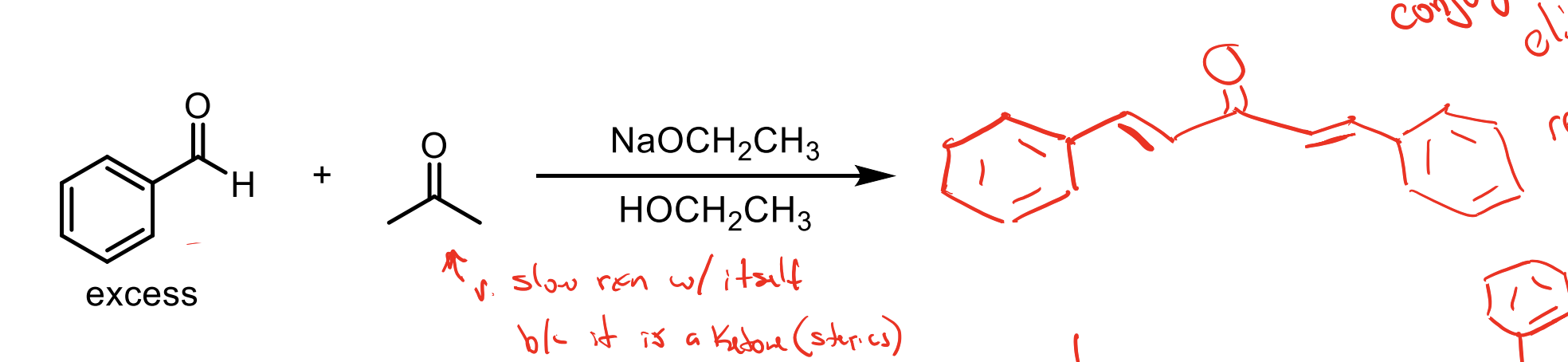
There is an excess of the electrophile. You will form the product with a beta unsaturated ketone almost immediately (because of the conjugation with the benzene ring)
So with your solution having NaOCH2CH3 and HOCH2CH3 you can make enolate again which will then react immediately with the excess of electrophile then form another beta unsaturated ketone.
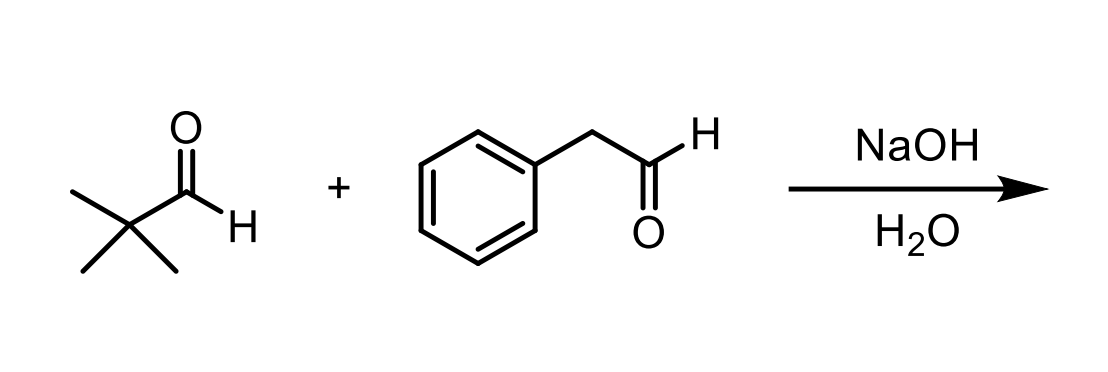
One has no alpha hydrogens the other does. You didn’t add the one with alpha hydrogens slowly so you’ll get two products.
You’ll get one where our nucleophile reacts iwth the tert butyl aldehyde thingy and that will be conjugated by the benzene ring so you’ll get the beta unsaturated ketone (E1CB).
You’ll get another that reacts the nucleophile with itself then since it still has conjugation with the benzene ring you’ll form the beta unsaturated ketone (E1CB).
When you do the E1CB mechanism, you eliminate the other alpha hydrogen from that carbon, so in order to actually kick off the OH you need another alpha hydrogen. This means if a molecule like the one in this had only one alpha hydrogen, we would just get the aldol product regardless. Pay attention to mechanisms.
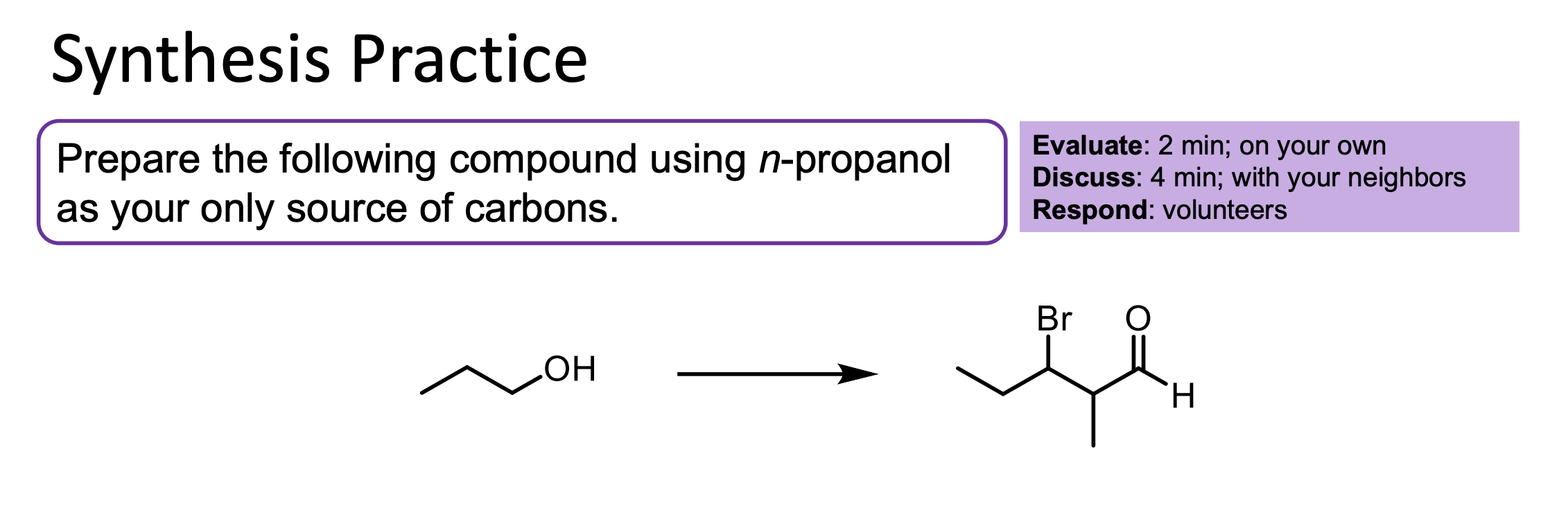
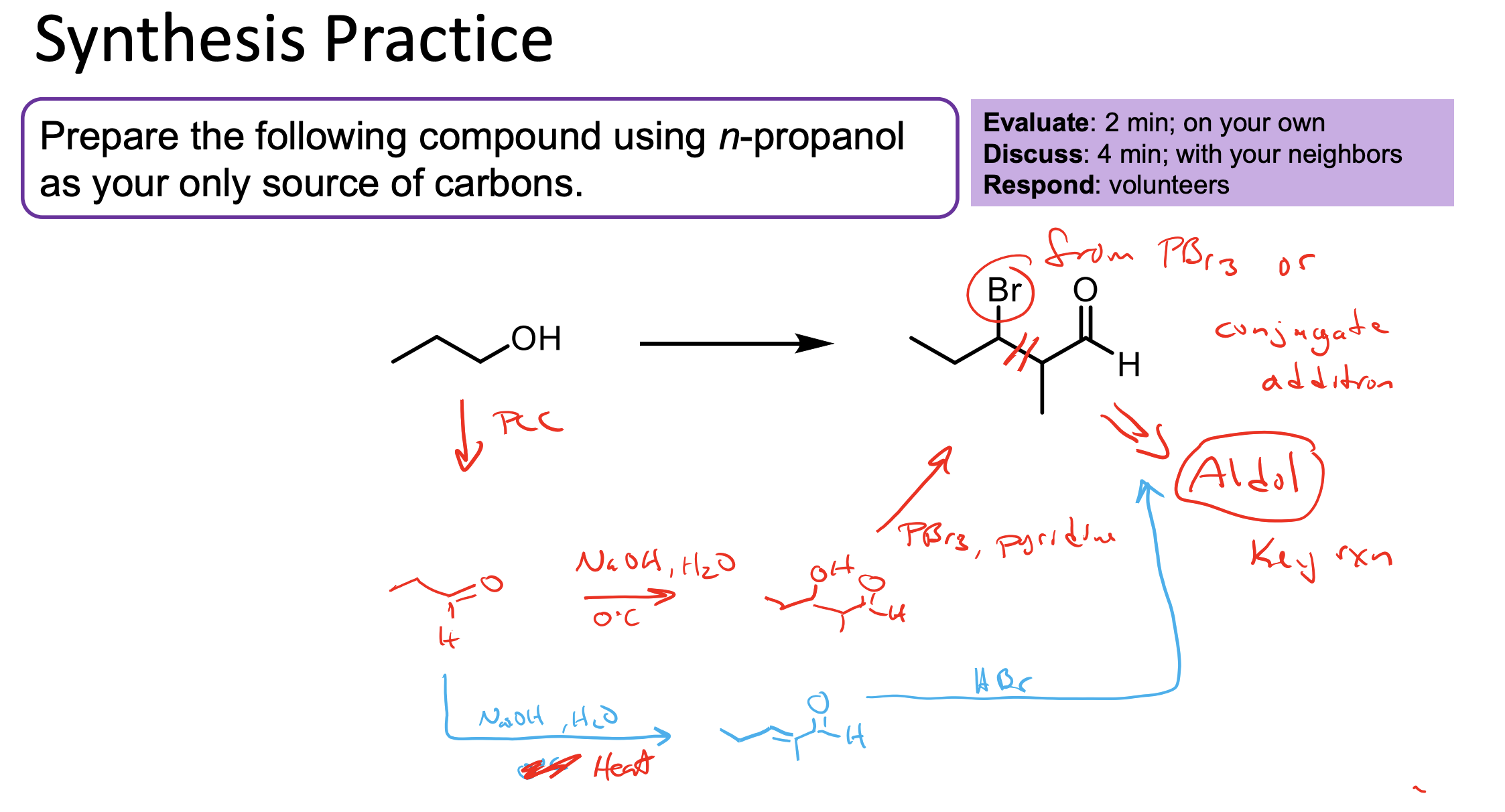
You use PCC to convert all of this alcohol to an aldehyde.
You use NaOH, H2O + ∆ to react it with itself to make the aldol, then the heat makes the beta unsaturated ketone which can be reacted with HBr to make our product (alkenes react with HBr).
2.1 You could also use NaOH, H2O, 0°C to just form the aldol product, then use PBr3 and Pyridine to convert the OH to a Br.
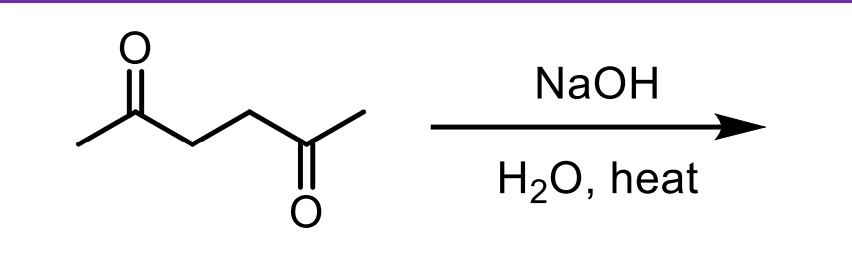
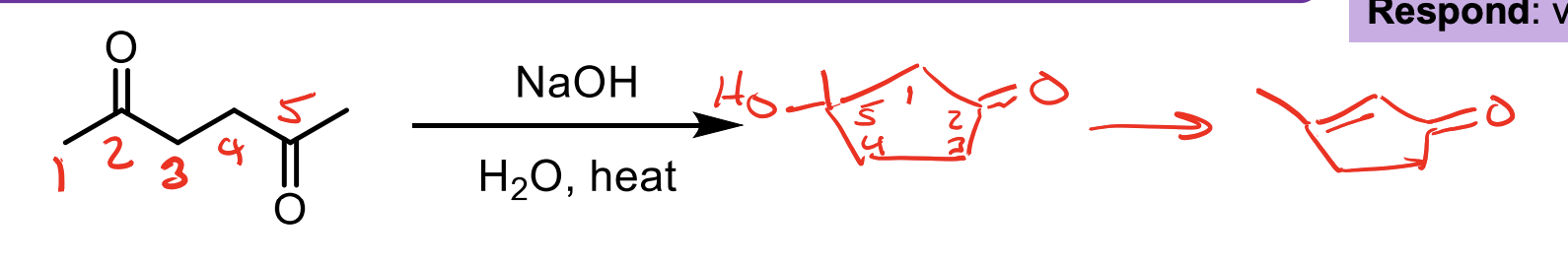
What is the thought process behind adding NaOH, H2O, and Heat to an aldehyde?
The NaOH and water will allow some of it to be converted to the enolate. This will allow it to attack itself (because most will still be ketone) and the water will protonate the O- formed to an OH. Now you’ll have a B-hydroxy aldehyde.
When you add heat you convert the beta-hydroxy aldehyde to a beta unsaturated aldehyde/ketone via the removal of the OH group via E1CB.
so if you have a carboxylic acid next to a ketone such that its carbonyl is in on the ketones beta carbon, if you apply heat it will eliminate it
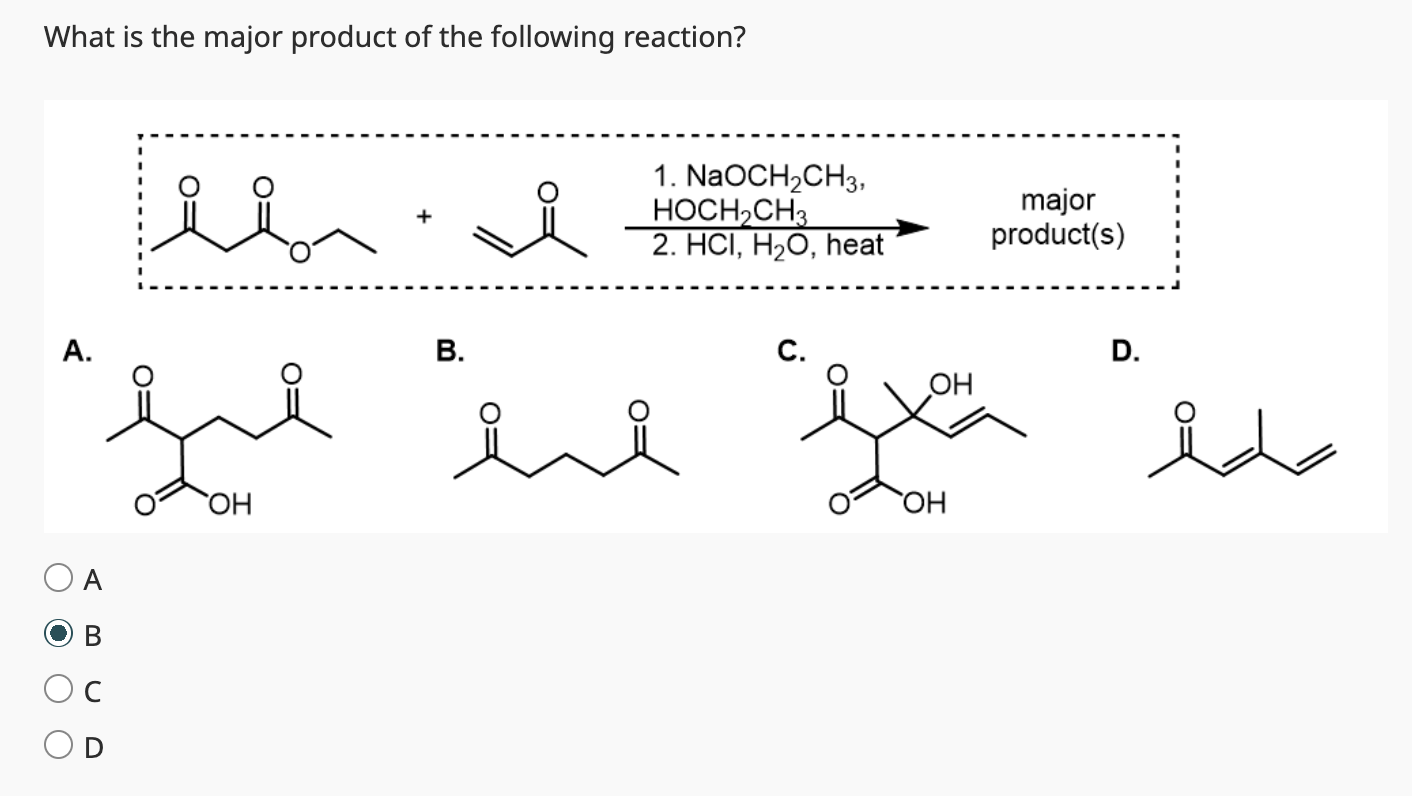
Explain how the Claisen Condensation reaction is reversible?
Once you add the other ketone (it will have pushed off the OR) you now have a di-ketone compound with HIGHLY acidic and unstable alpha hydrogens in the middle. (pKa of di ketone ester is 10.7 and the pKa of an alcohol is 17 so it is way more likely to have the OR- protonated to the alcohol).
This means the compound exists more in its enolate ion form until you add H3O+ to protonate it to the di ketone again.
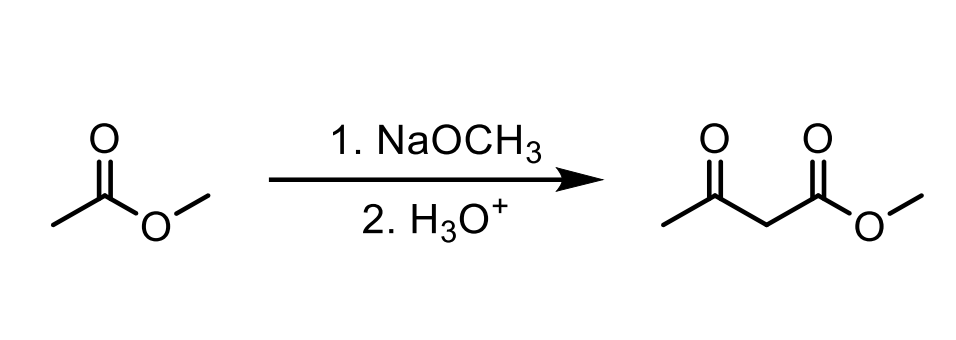
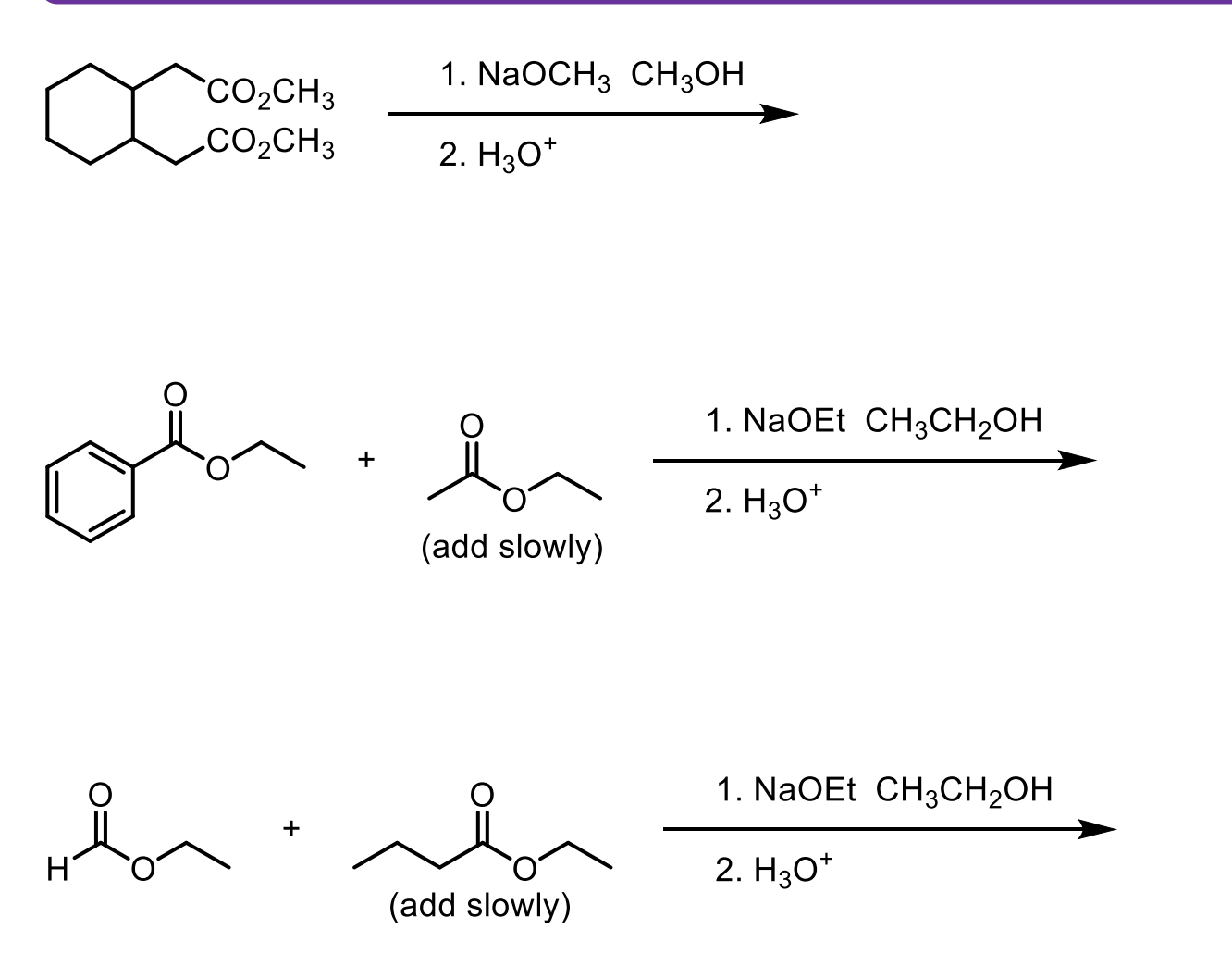
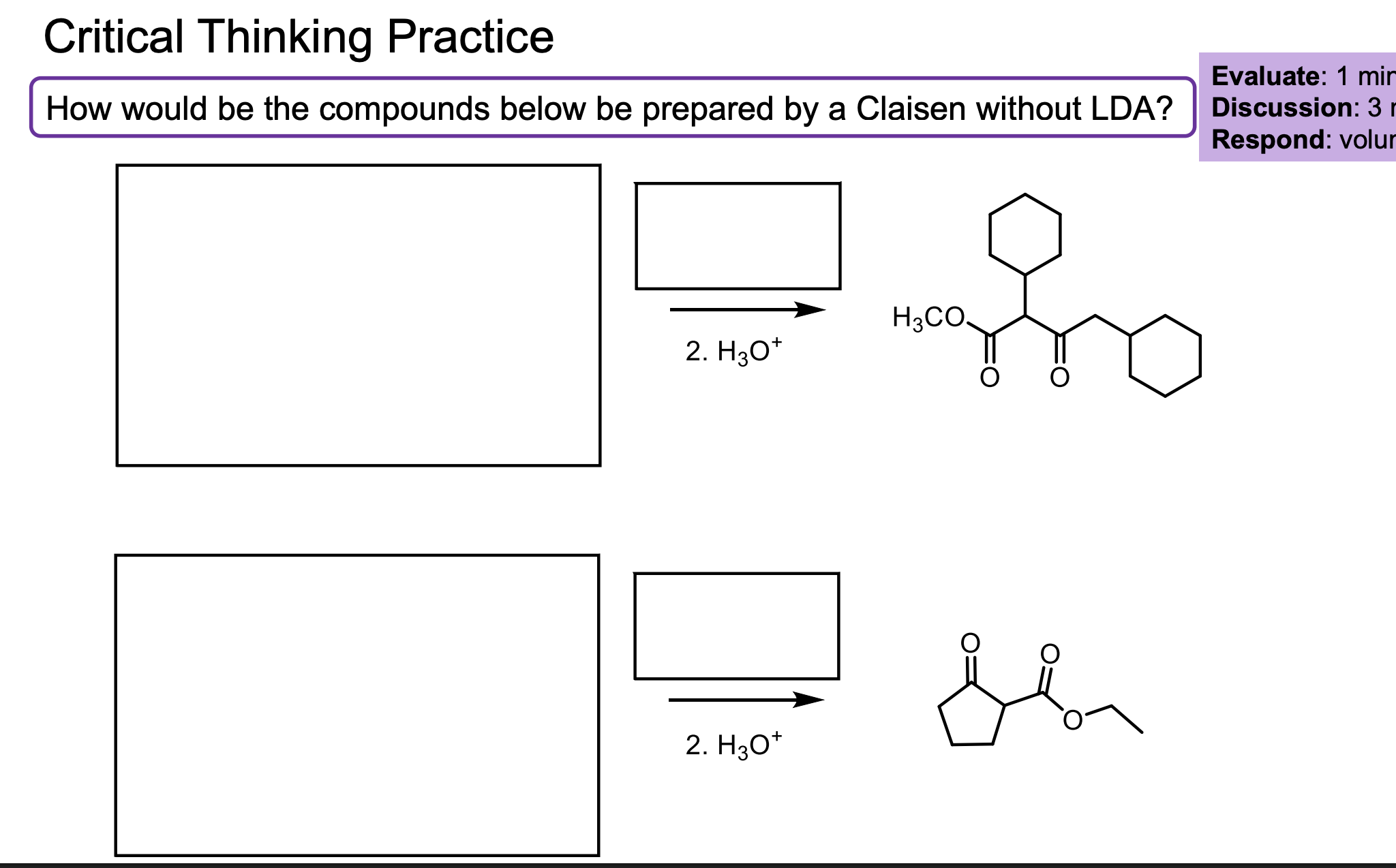
Walk through the Claisen Condensation Mechanism
Don’t forget the step where you deprotonate to the enolate then add H3O+ to make it the product again.
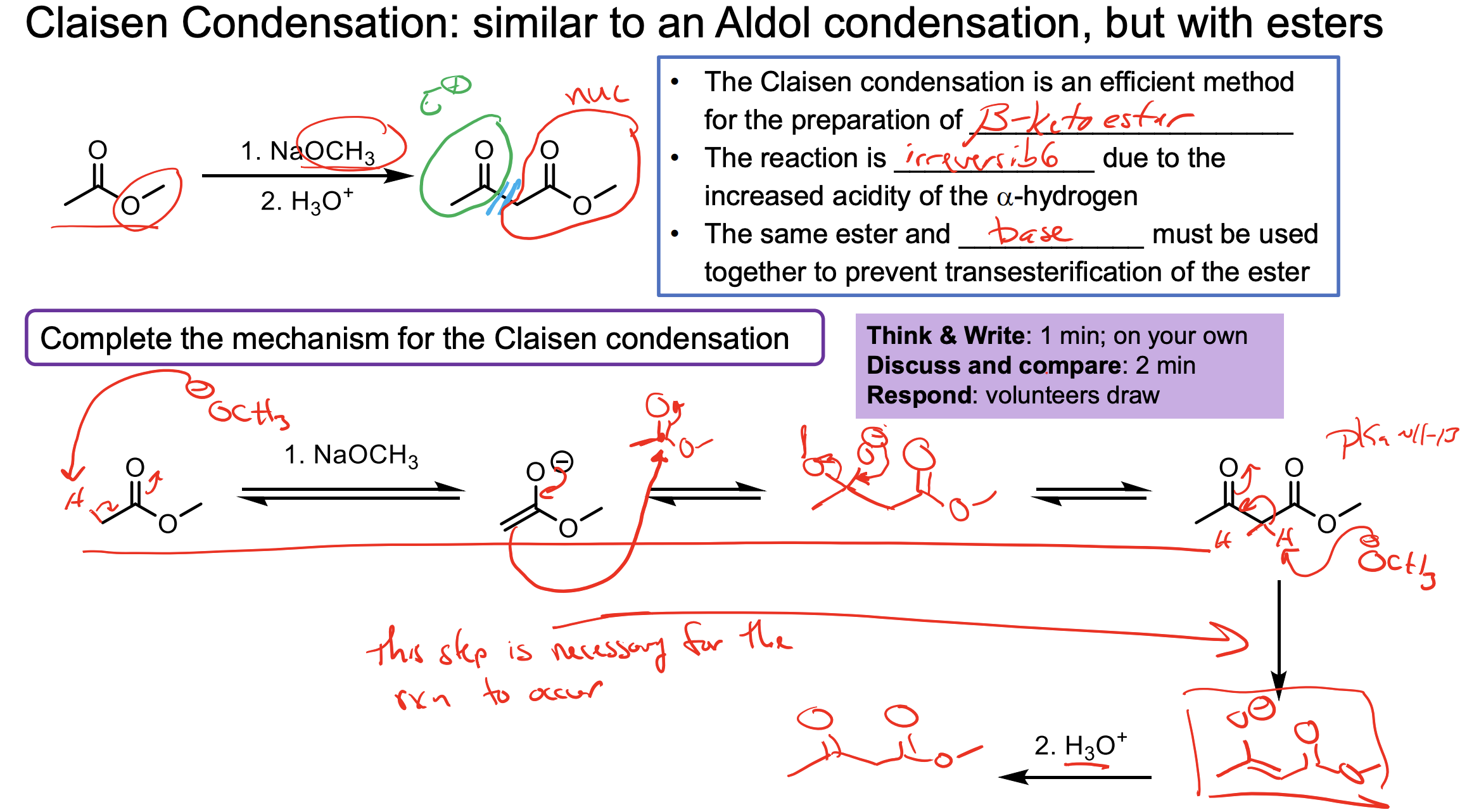
If the R group does not match the ester, it will undergo transesterification which will then cause that molecule to react with itself.
This is why we need the R groups on the ester to match.
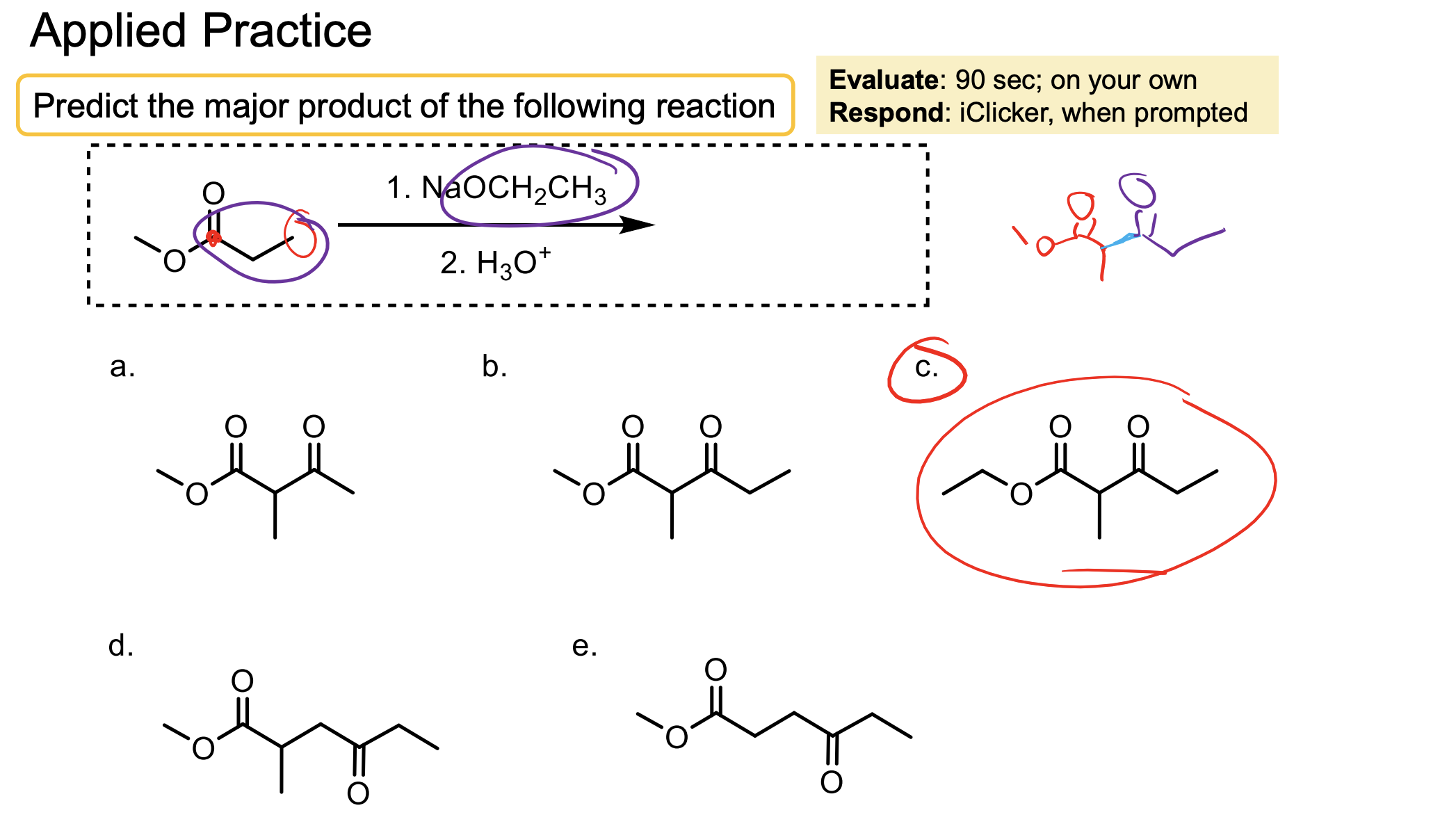
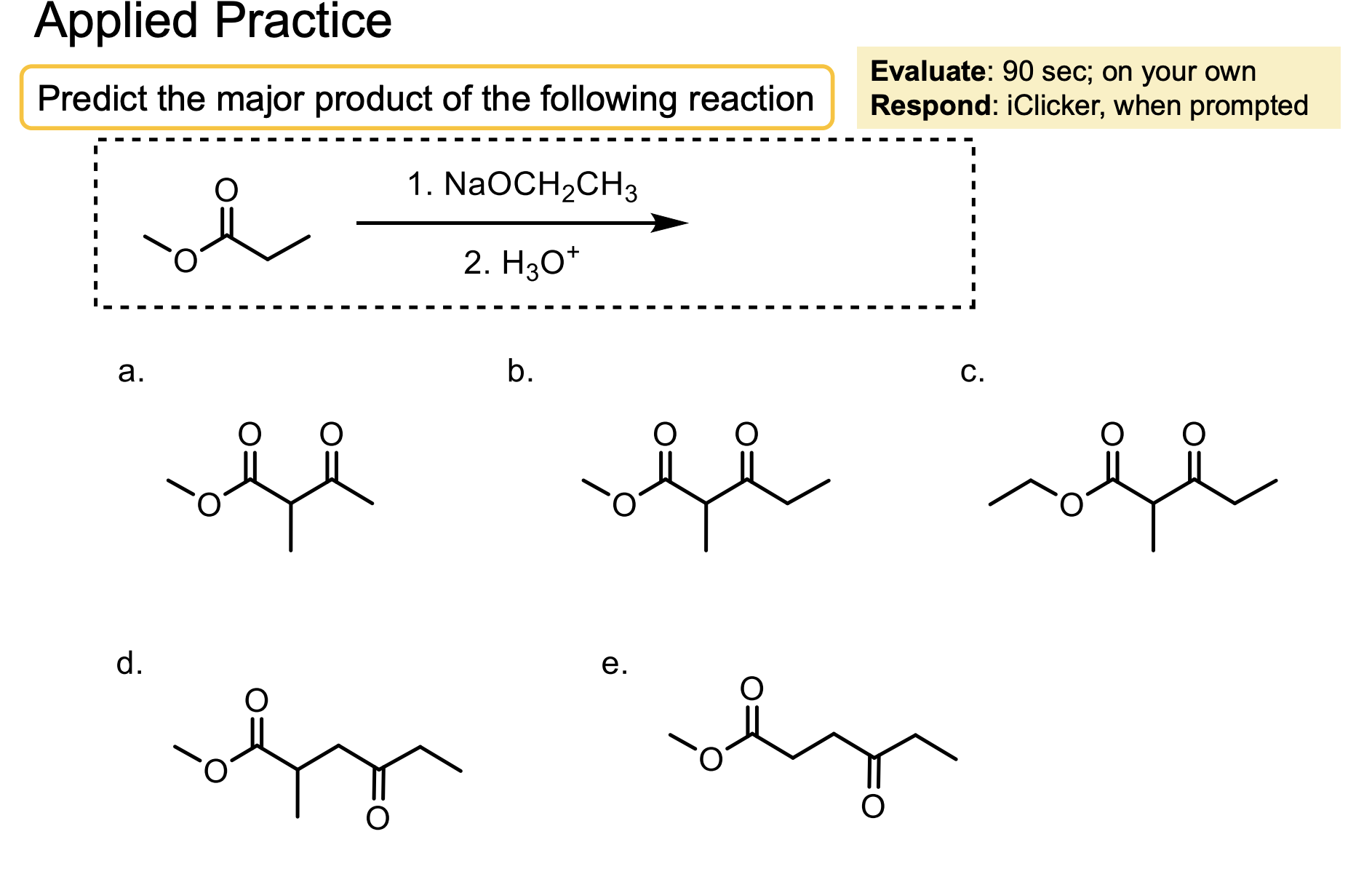
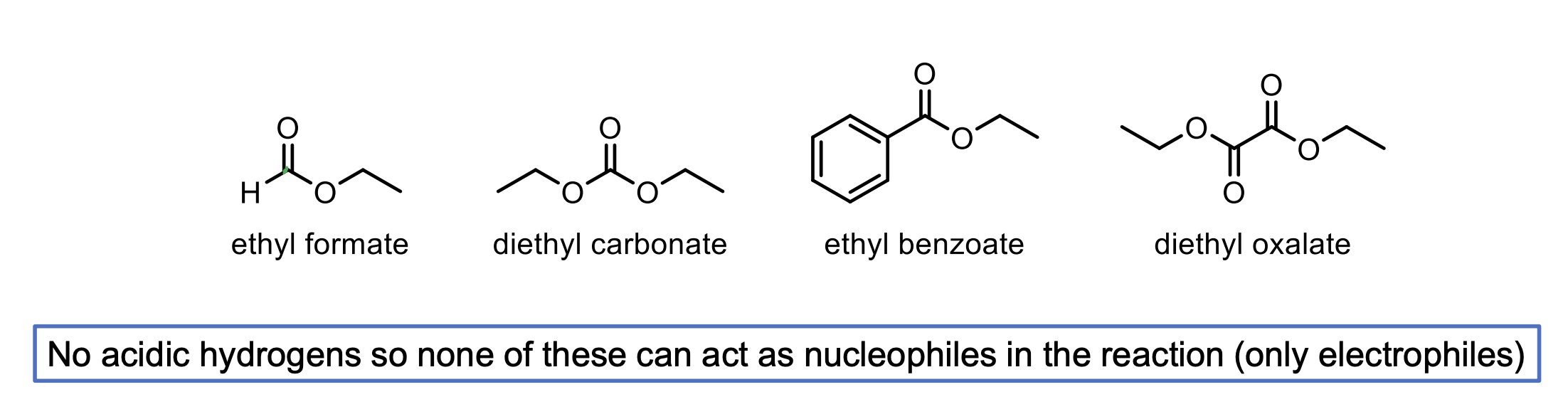
What are some examples of molecules that can be used in Crossed-Claisen Condensation that can only act as electrophiles? Why is that?
These molecules don’t have alpha hydrogens.
Ester with Hydrogen (ethyl formate)
Ester with another OR (diethyl carbonate)
Ester with benzyl ring (ethyl benzoate)
Ester with another ester directly attached (diethyl oxalate).
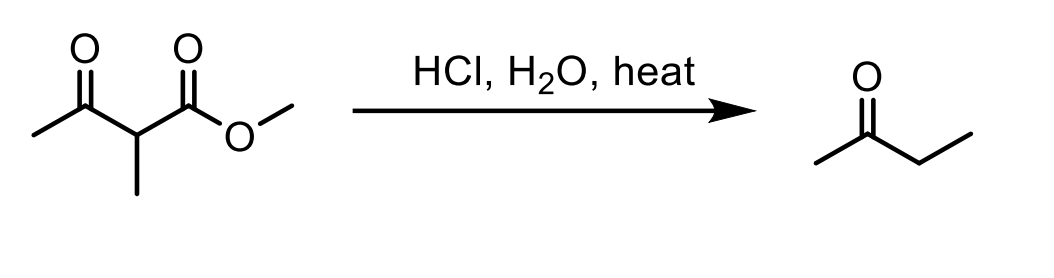
What happens when a beta keto ester (ketone right next to an ester on its beta carbon) is reacted with HCl, H2O, Heat? Draw the mechanism.
HCl, H2O, Heat convert the ketone to a carboxylic acid.
The pi bond of the ketone deprotonates the highly acidic carboxylic acid Hydrogen, then the O-H bond pushes to form a O=C=O which pushes the C-C bond to the carbonyl forming an enolate. Just look.
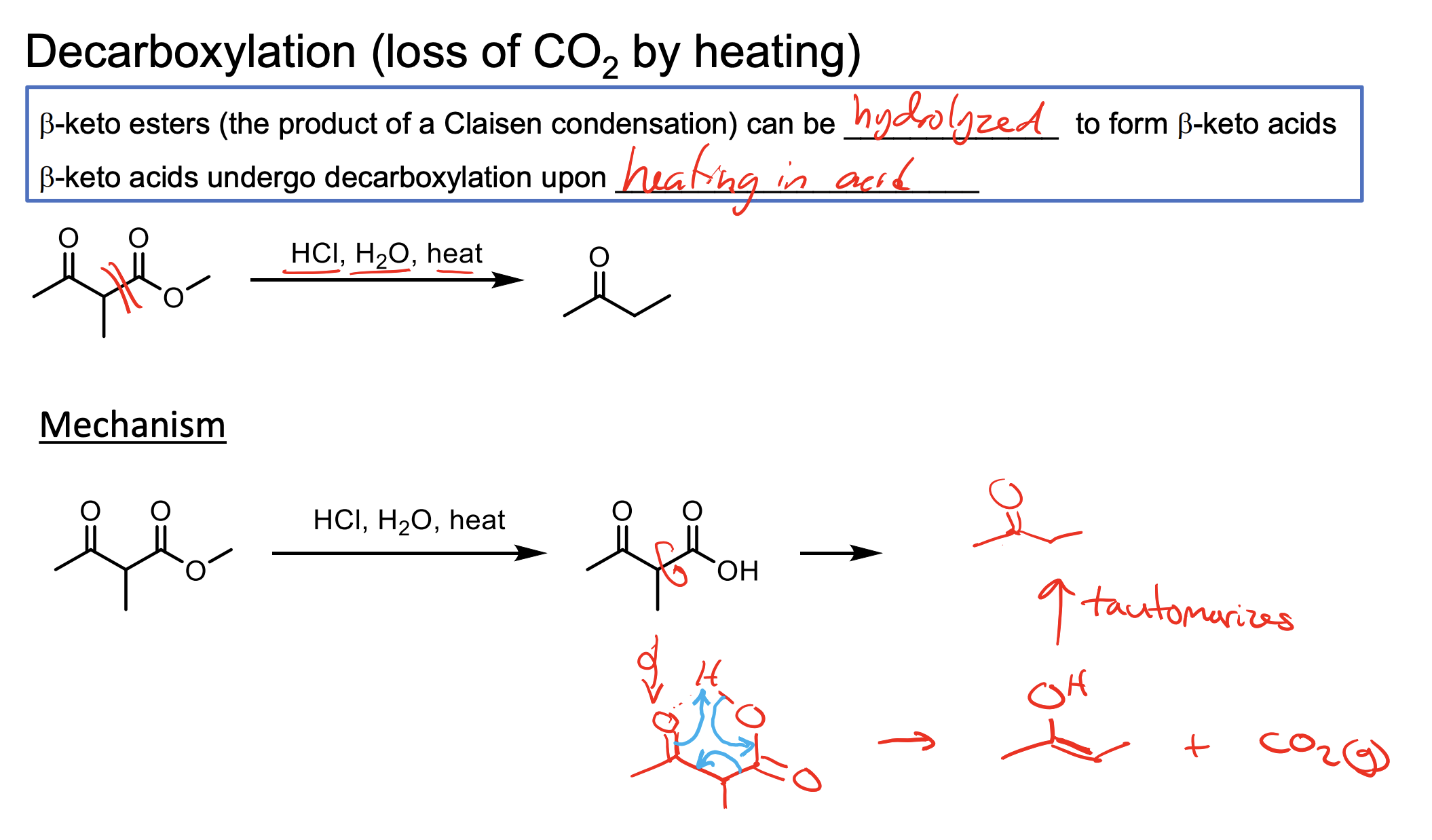
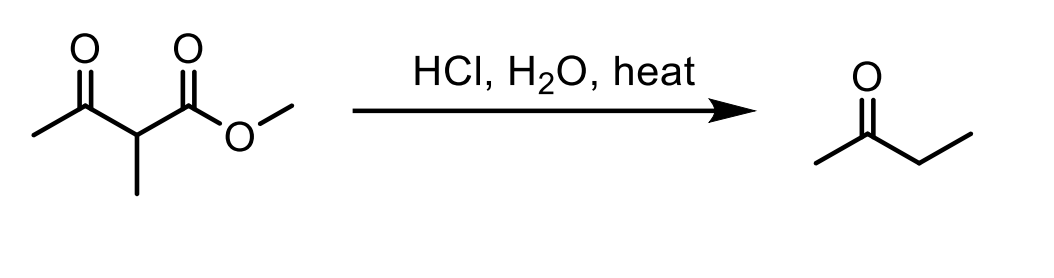
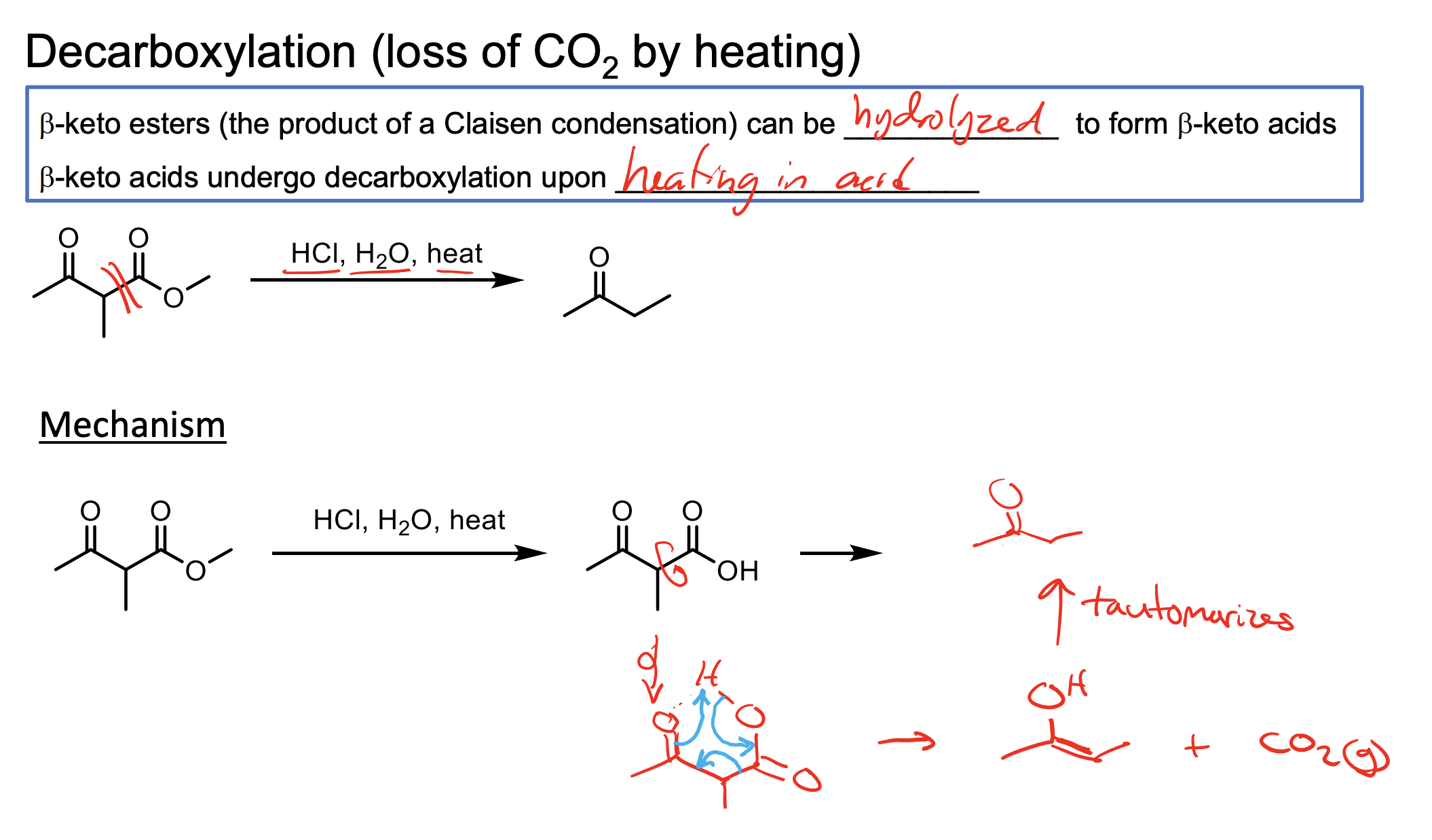
Remember it has to be a beta keto ester so you need that and H2O, HCl, and Heat ∆ to make this happen!
Why do we need that second step?
Remember in the reaction we actually form the product then the -OR that we push off deprotonates the very acidic middle alpha hydrogen forming an enolate, which we need the second step to reprotonate.
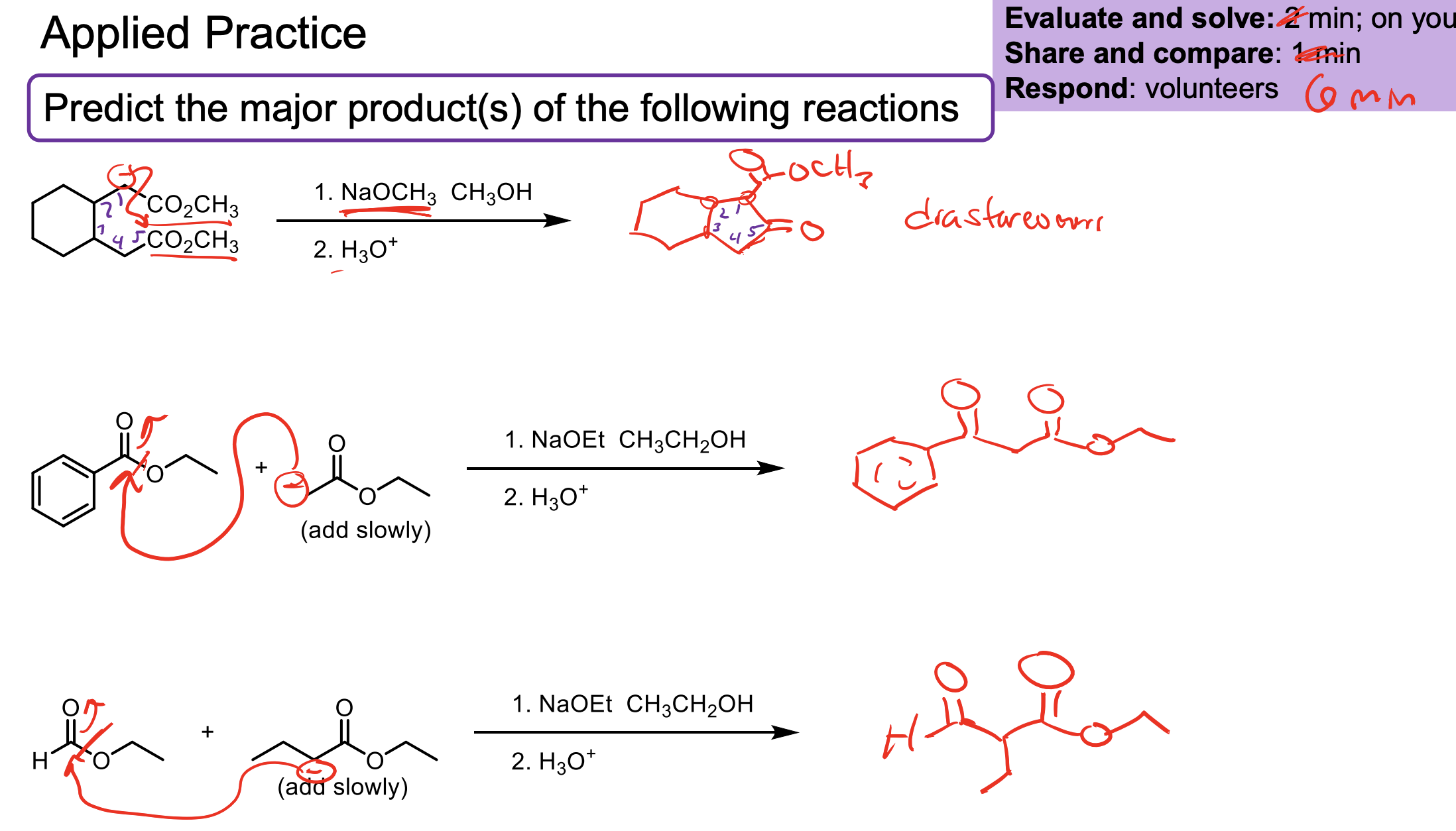
Remember you want that OH not another ketone so your electrophile must be an aldehyde/ketone which means you have to follow up its attack with an H3O+ w/u.
You can add LDA to only your reagent to make it all enolate then add the aldehyde slowly to get it all to react.
Then follow it up with an H3O+ w/u.
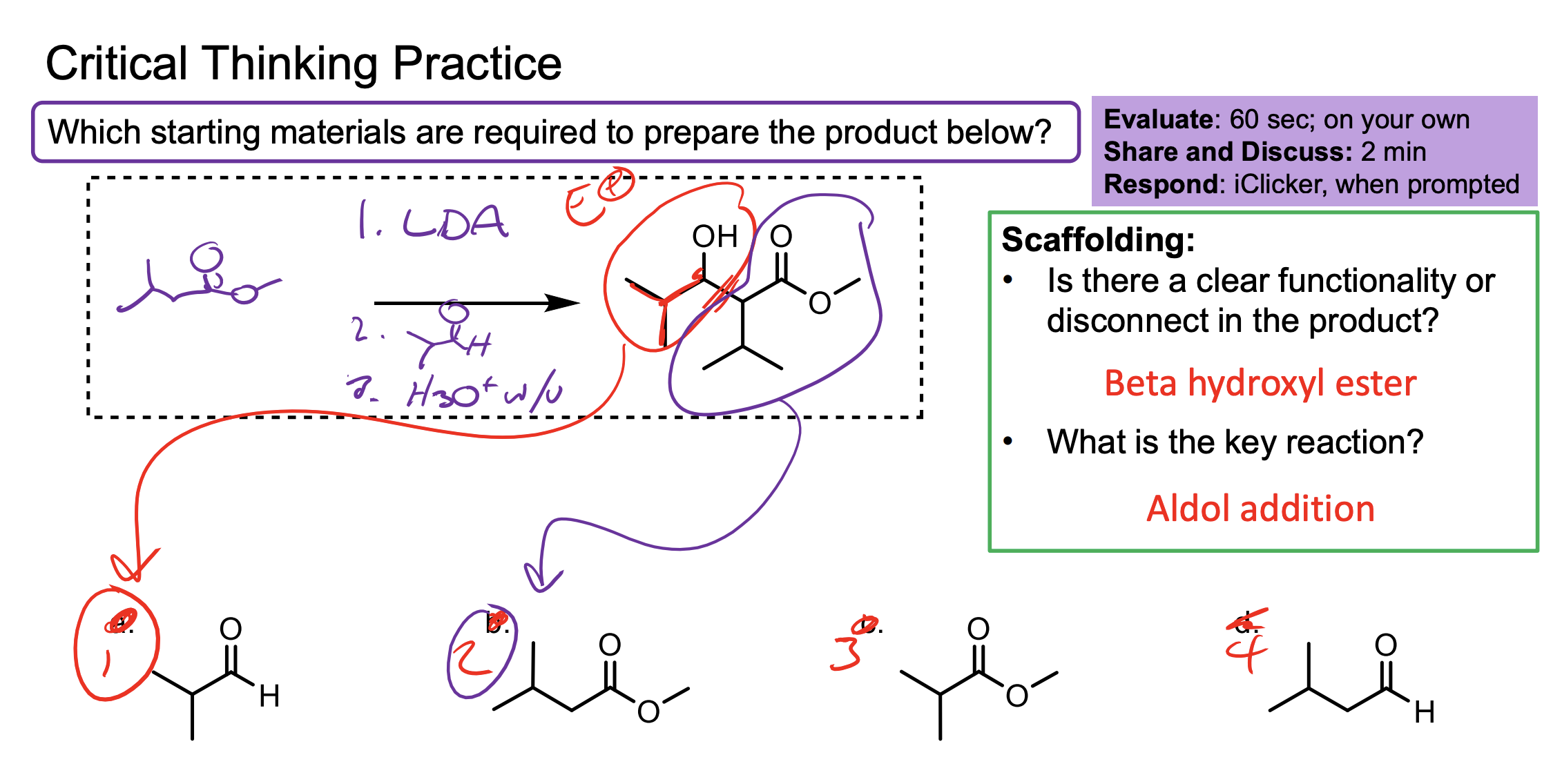
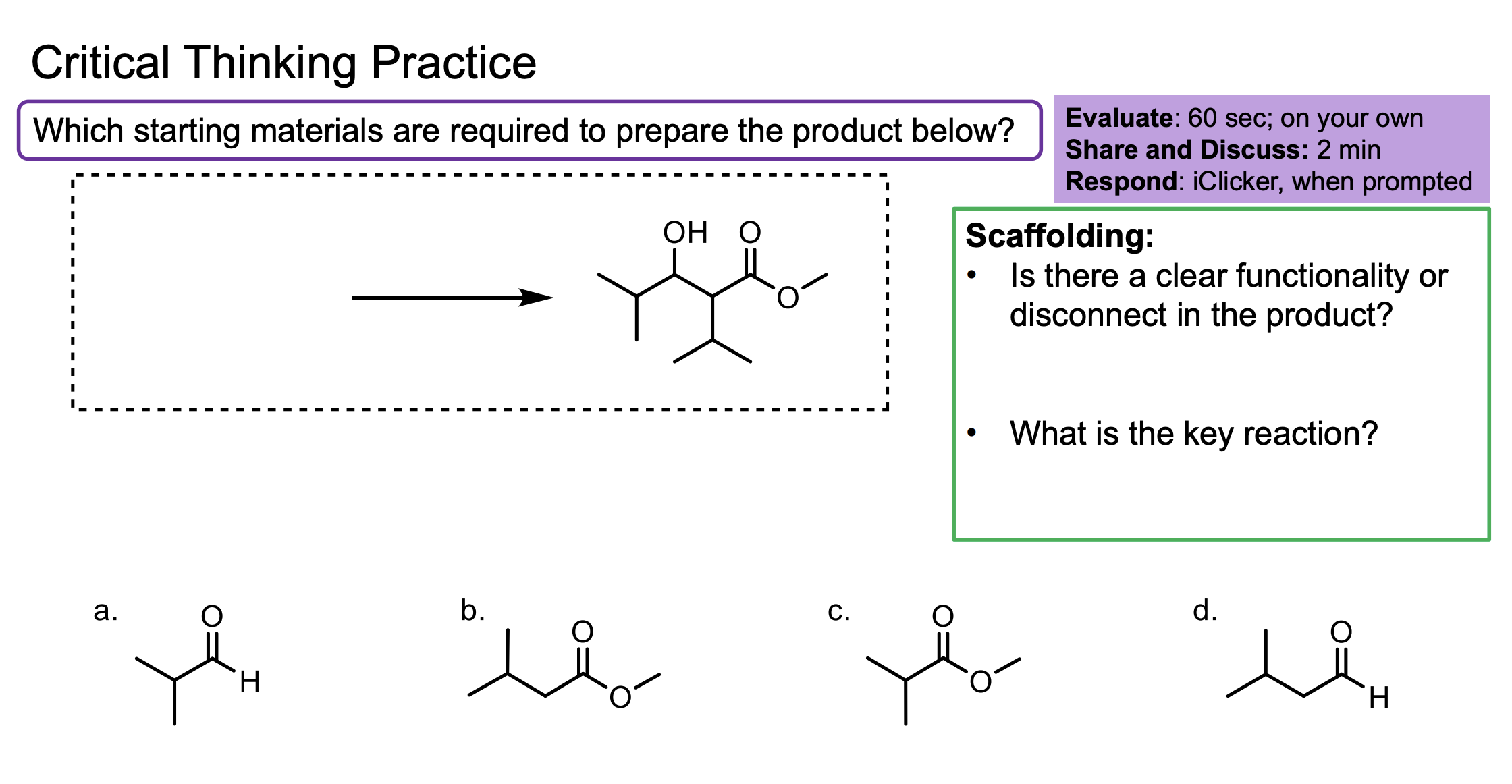
Make sure you count the carbons on the first one don’t mess up counting that’s lame.
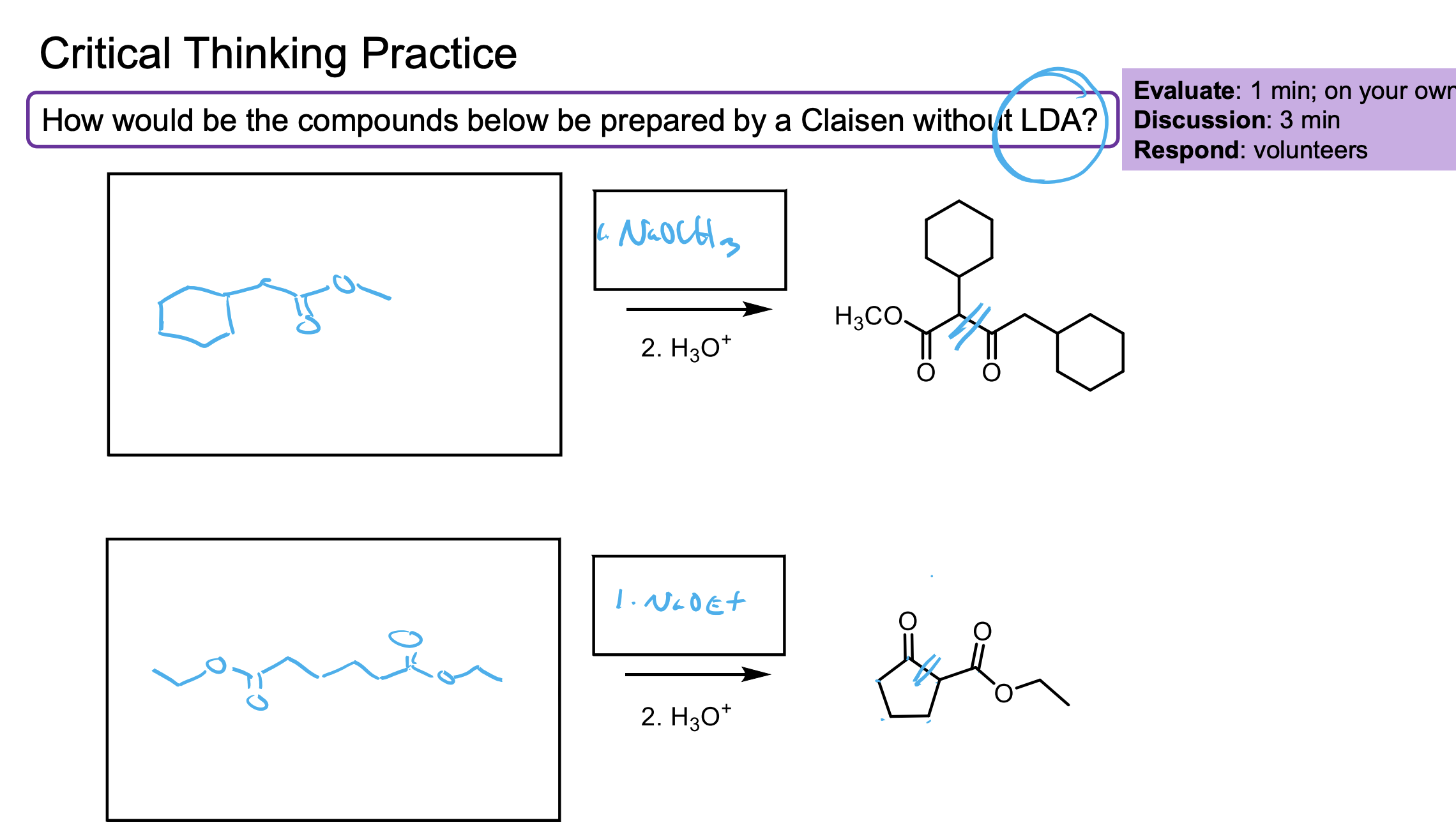
Remember this needs the second step of H3O+ w/u becuase you form the enolate which then needs to be prorontated after it kicked off the -OR because the -OR is what makes it the enolate.
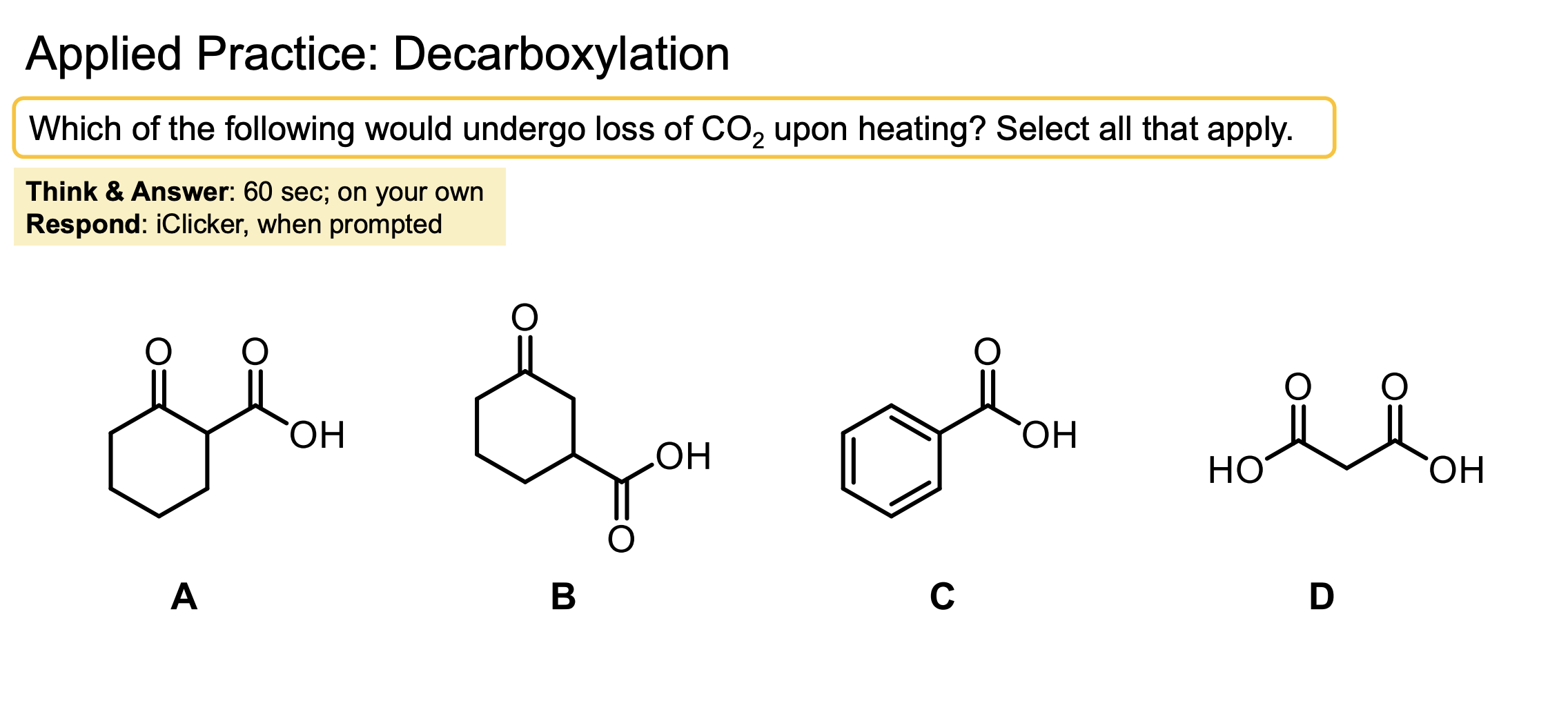
An ester with H2O, HCl, and Heat converts the ester to a carboxylic acid which then does decarboxylation (loss of CO2 by heating) please refer to mechanism as it is a bit tricky.