atomic mass #s and shiz
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
Atom
the smallest particle into which an element can be divided and still be the same substance
Atomic Number
the number of protons in the nucleus which gives the identity of the element

Electron
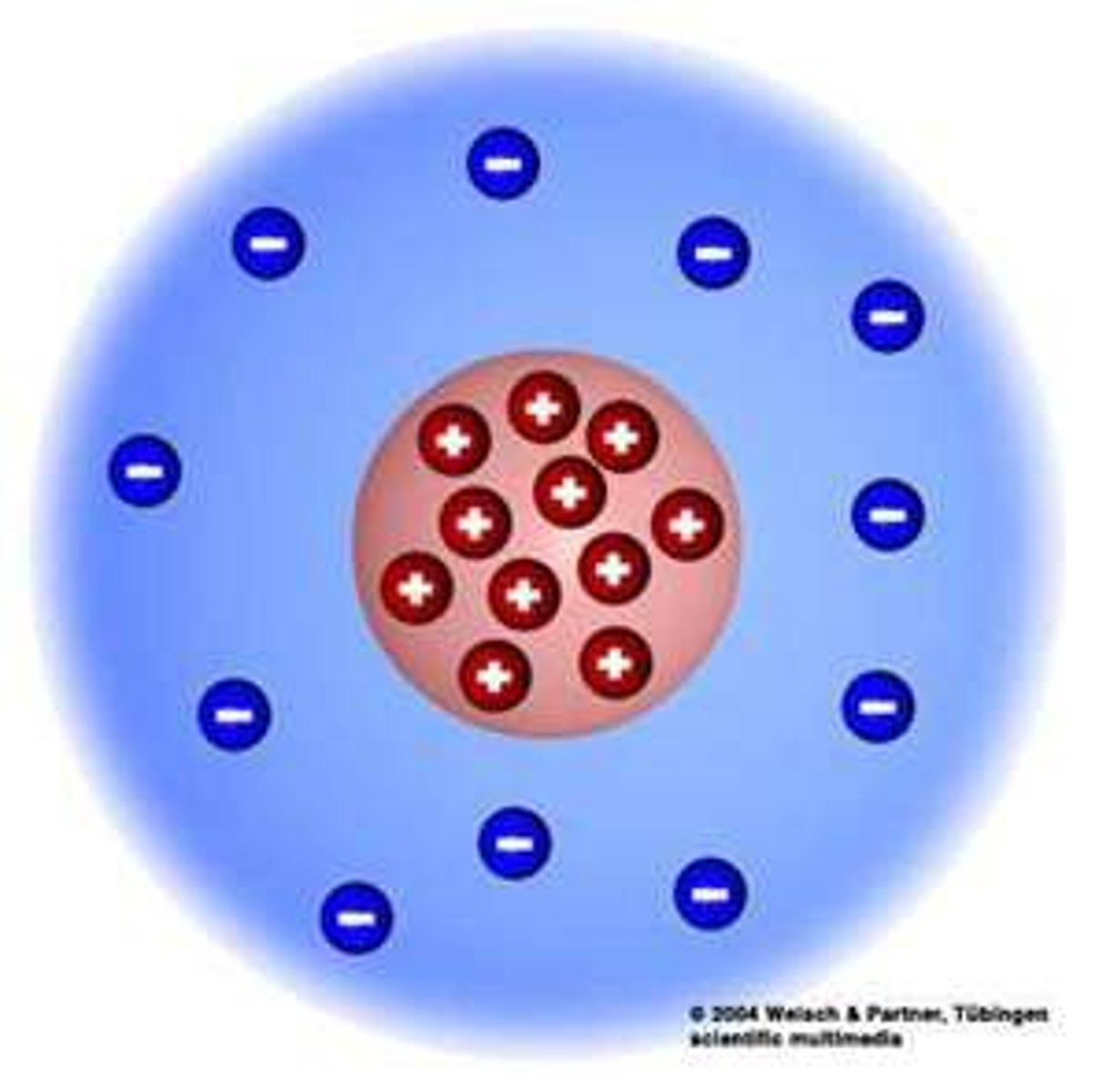
negatively charged particle that orbits the nucleus of an atom
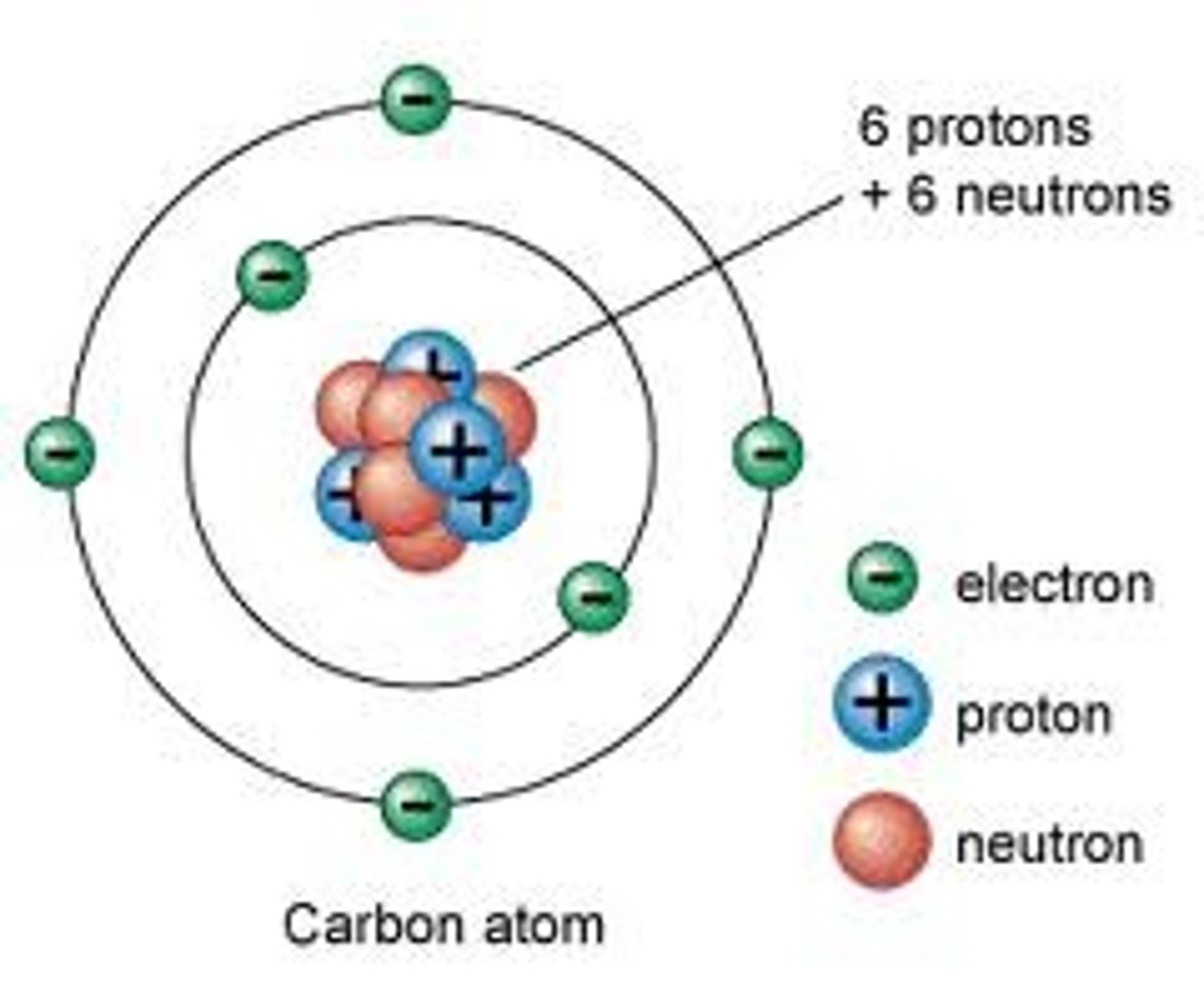
Electron Cloud
in the electron cloud model of the atom, region around the nucleus where an electron may be found
Element
a pure substance make of one kind of atom
Matter
anything that has mass and takes up space (volume)
Neutron
the particle with no charge; located in the nucleus
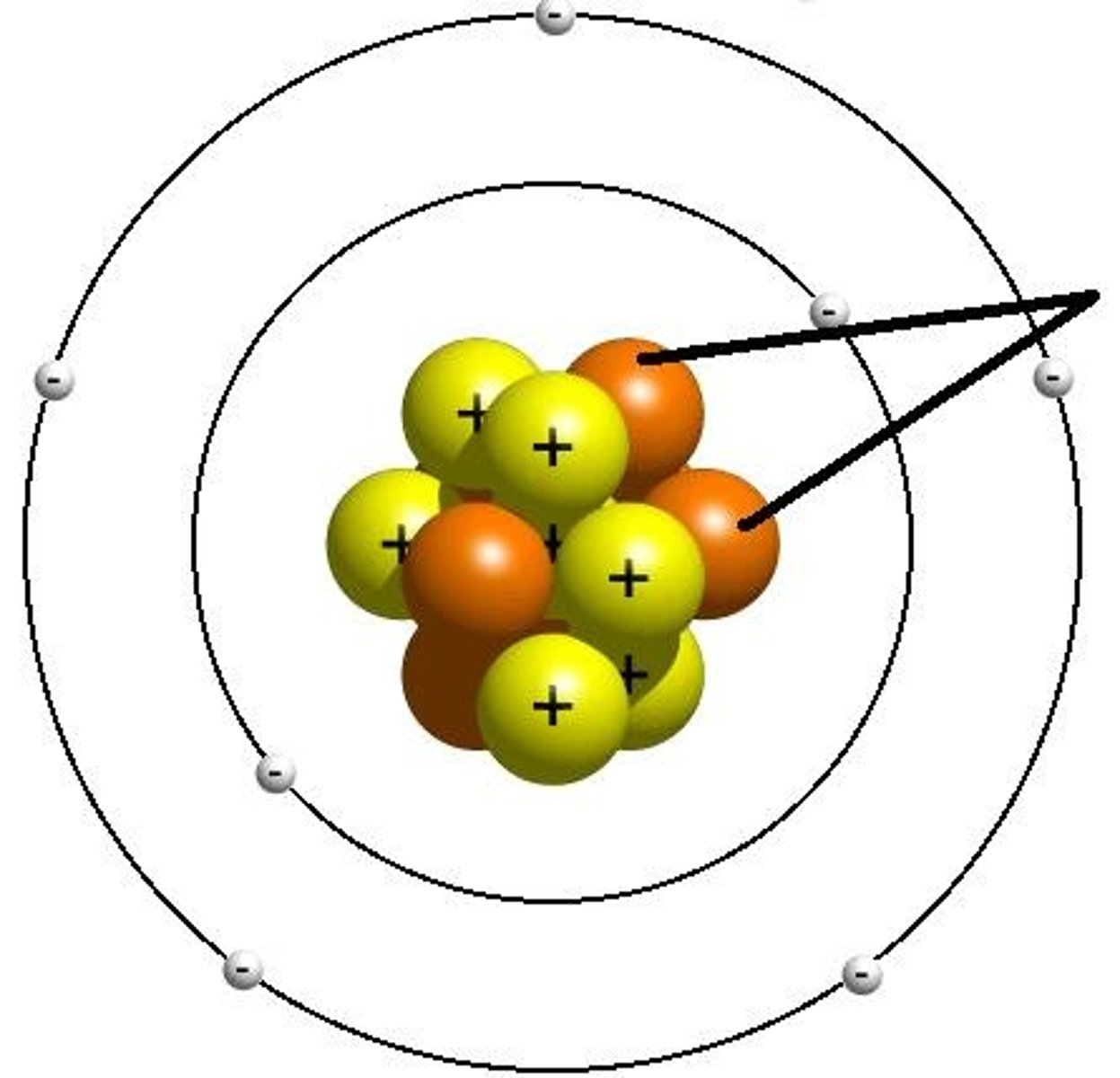
Nucleus
The center core of an atom that contains the protons and neutrons
Periodic Table of Elements
a chart where all the elements are organized into periods and groups according to their properties
Proton
positively charged particle located in the nucleus of an atom
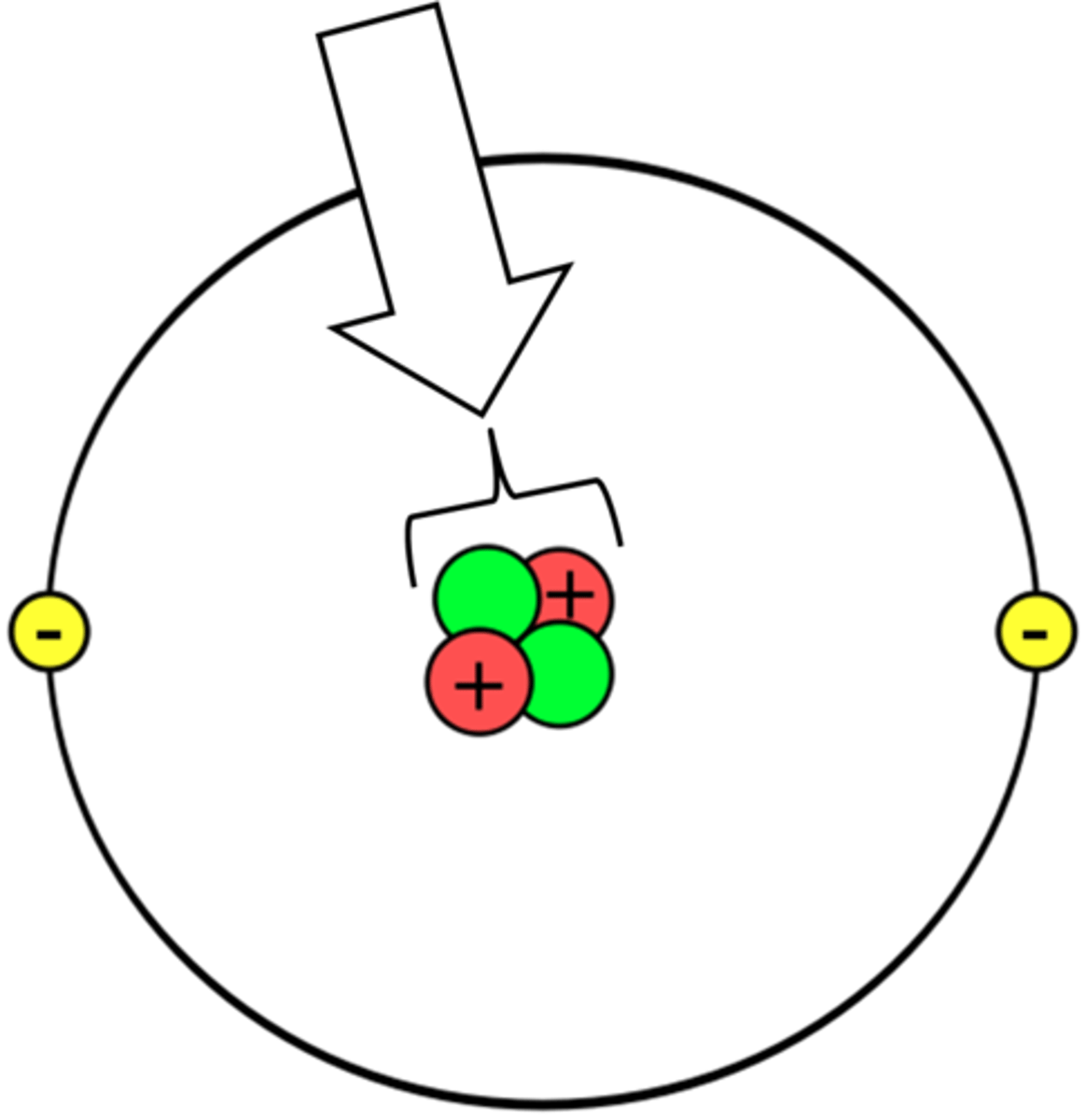
proton, neutron, electron
What are the 3 subatomic particles in an atom?
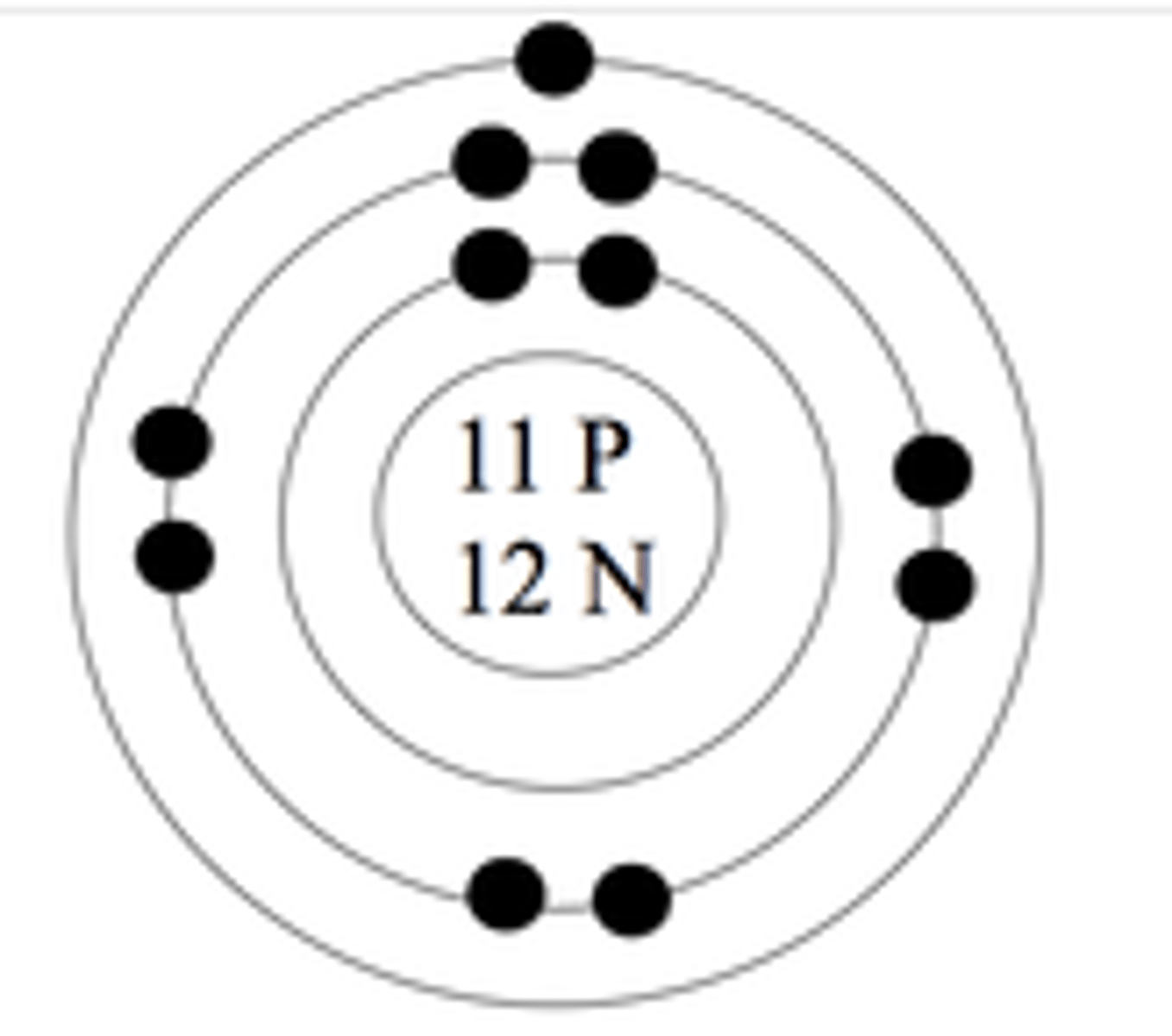
Sodium
Identify the element from the following information:
11 protons
12 neutrons
11 electrons
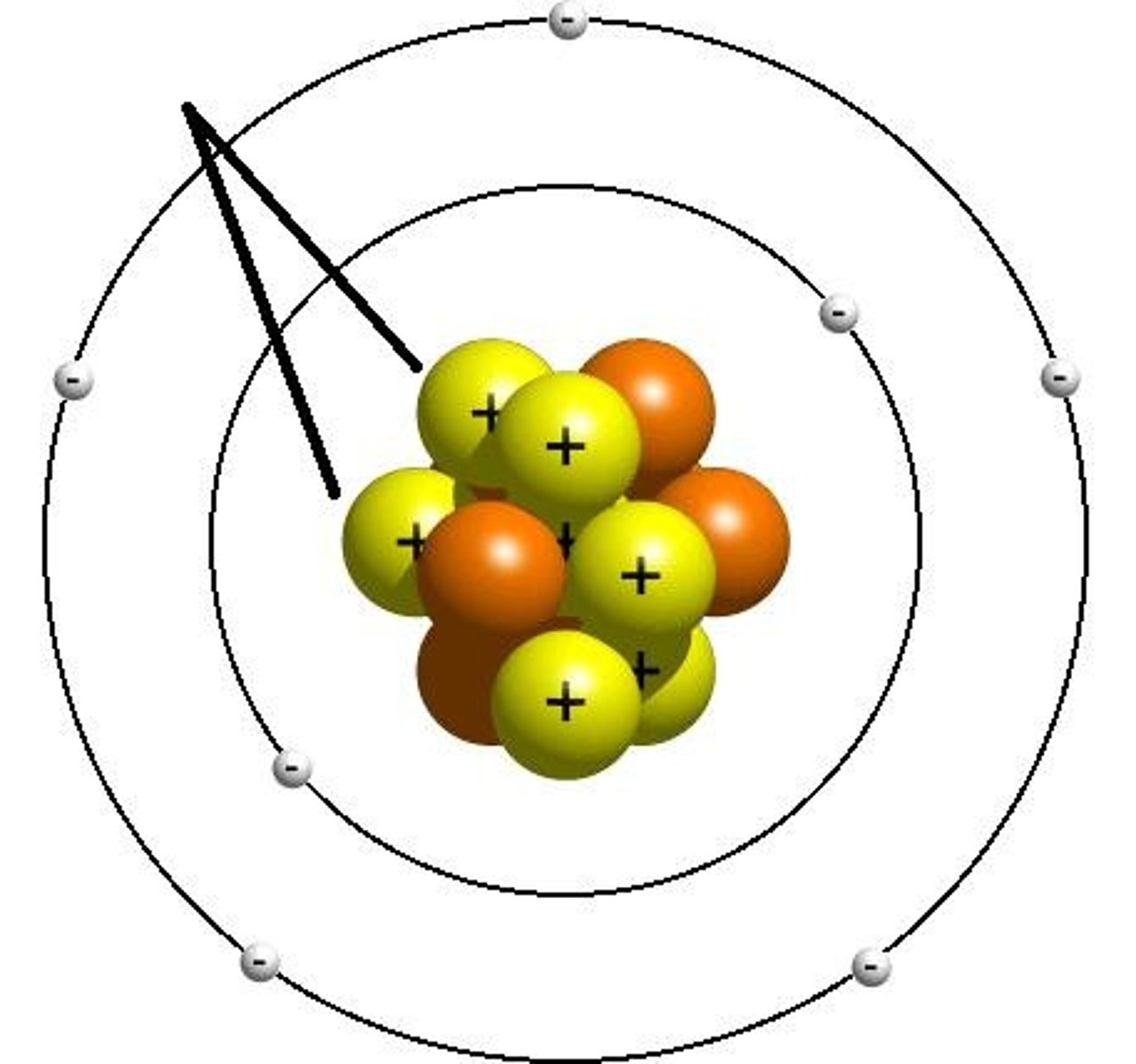
positive
What is the charge of the nucleus?

by the protons
How do scientists identify an atom?
protons and neutrons
What gives an atom its mass?
protons and electrons
A balanced (neutral charged) atom has equal numbers of ____ and _____
add protons and neutrons
How do you calculate Mass number?

atoms
What are the building blocks of matter?
nucleus
Which part of the atom is the most dense?
electron cloud
Where are electrons found?
Part of atom with most of the mass
nucleus
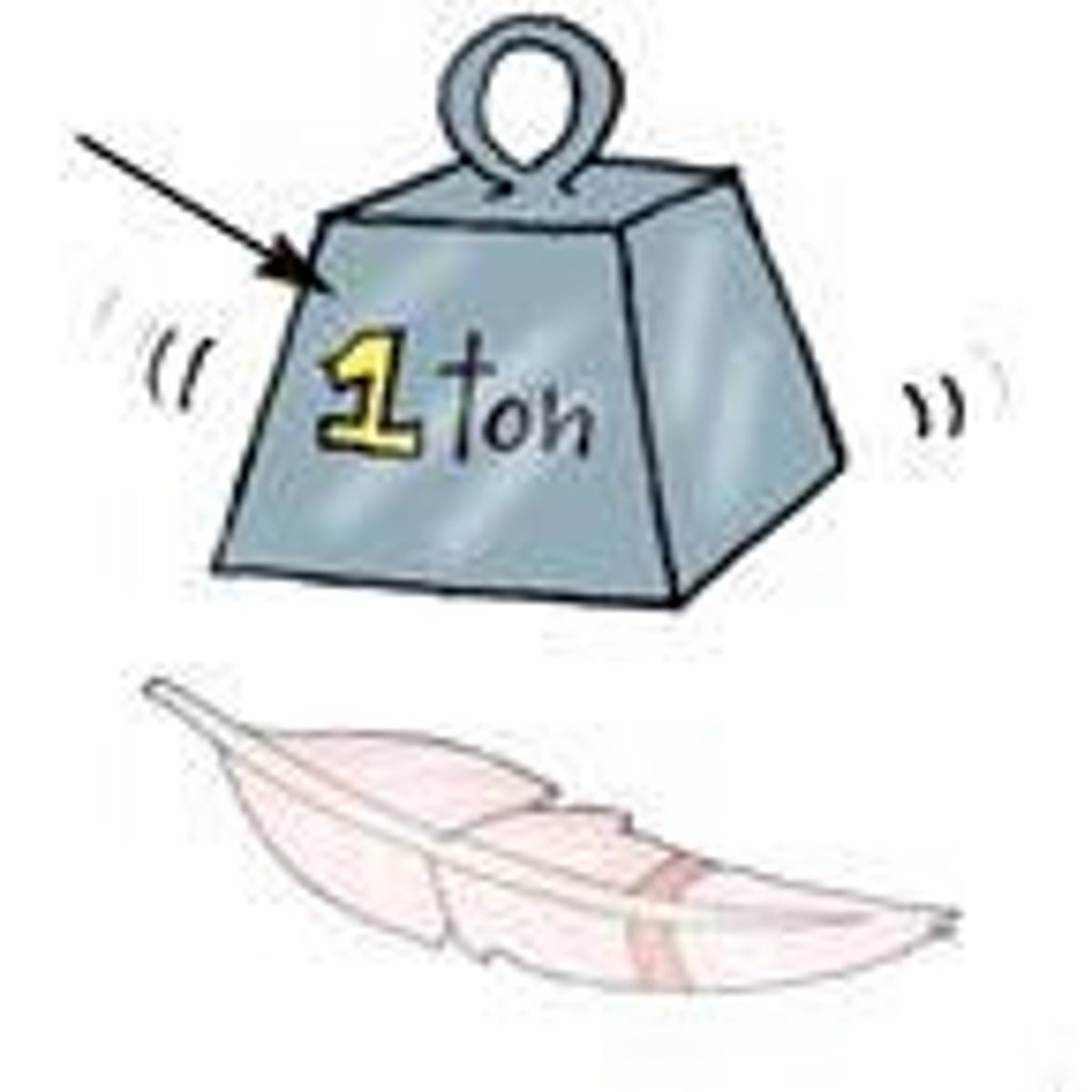
1 amu
What is the mass of a neutron?
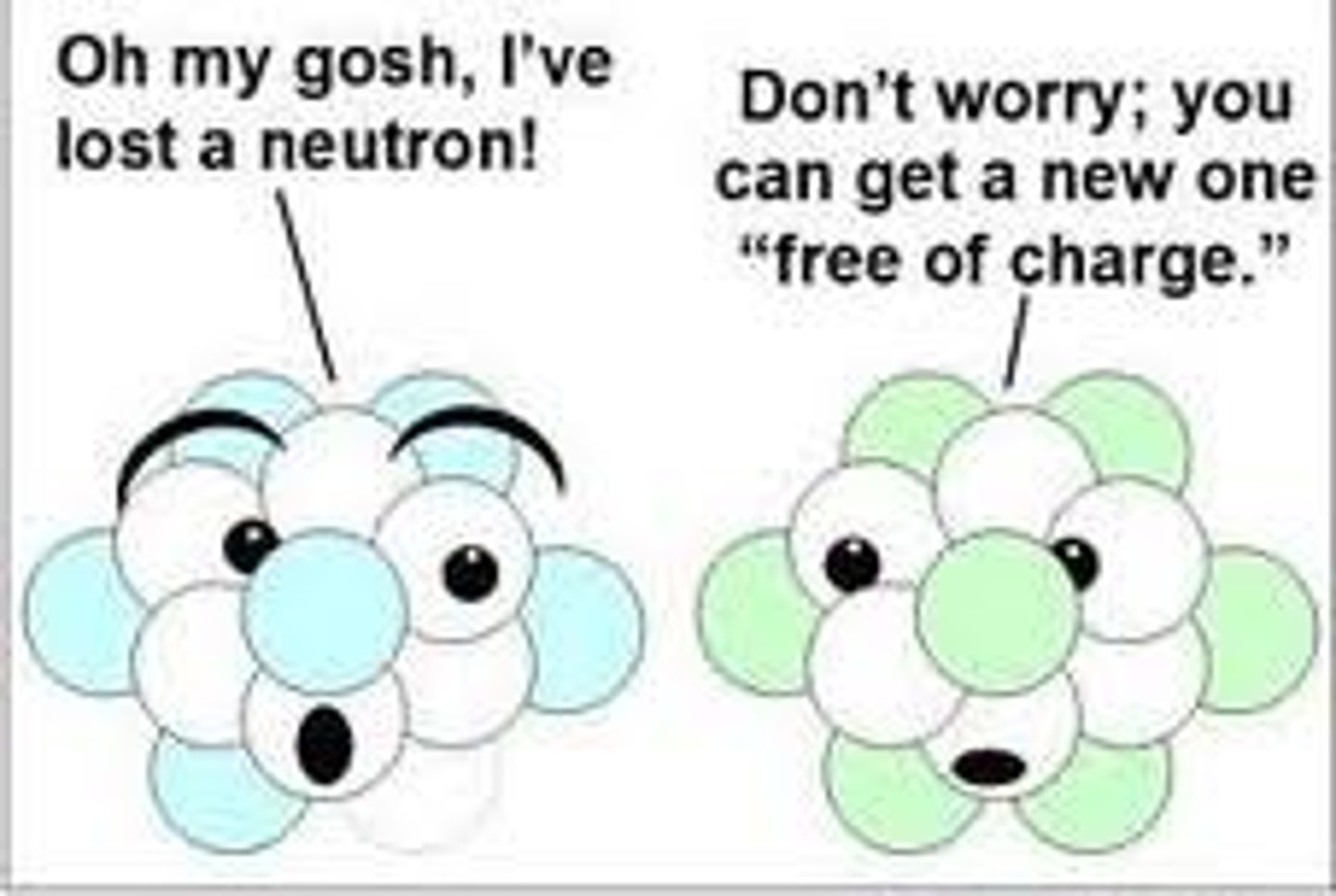
10
How many neutrons does oxygen-18 have?
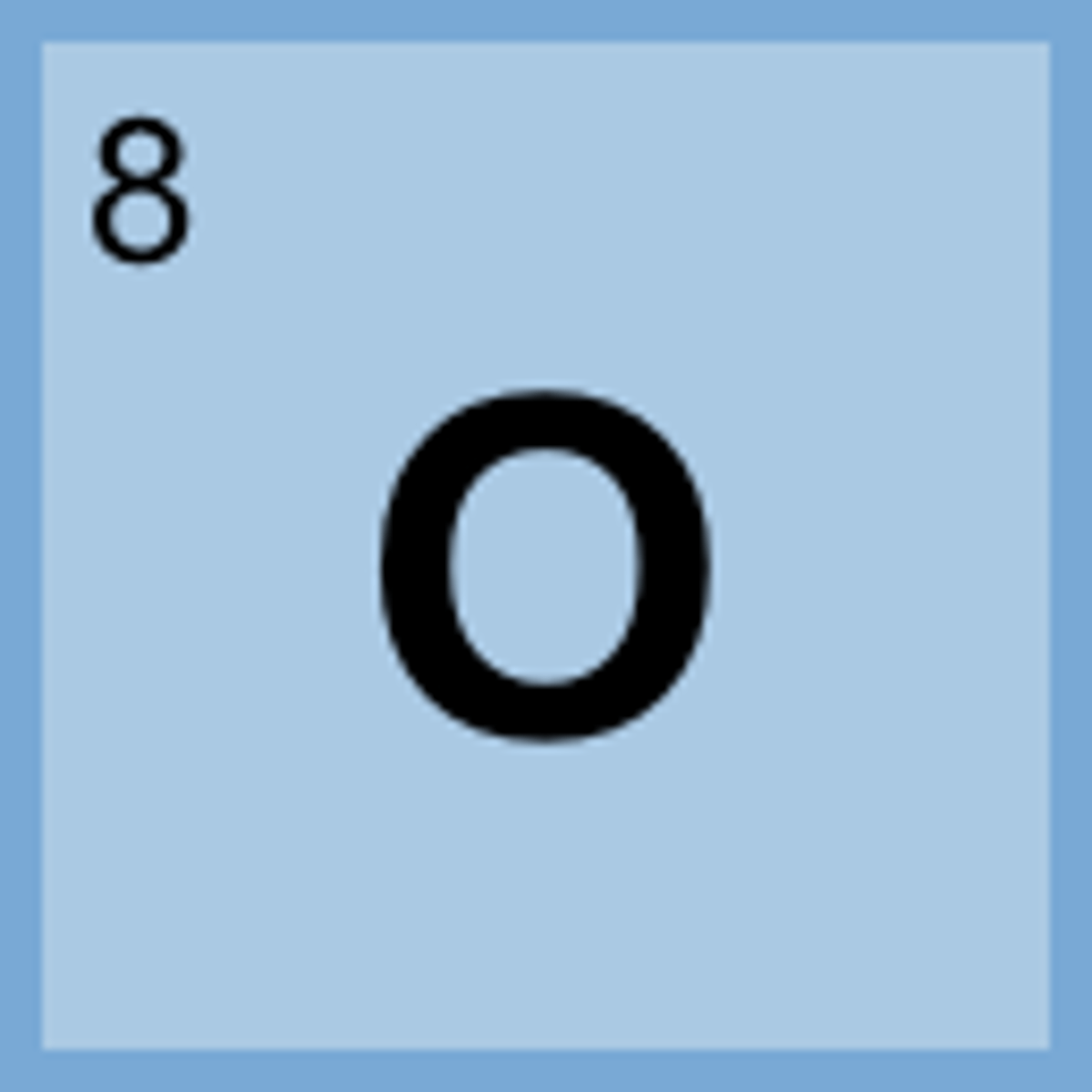
Is it possible for Nitrogen to have 7 protons, 8 neutrons, and 7 electrons? If so what is it called?
Yes--the protons determine the type of atom, Not the neutrons or electrons, those can vary

How many protons does Einsteinium have?
99

How many protons does Molybdenum have?
42

What is the approx. atomic mass for Iron?
56

What is the atomic symbol for Gold?
Au

How many protons does Neon have?
10

How many electrons does Silicon have?
14

Compound
Substance containing atoms of two or more elements chemically combined.
Mixture
Two or more substances which are physically combined and can be separated by physical means.
Atomic# 1
Mass# 1.008
Hydrogen
Atomic# 2
4.002602
Helium
Atomic# 3
Mass# 9.941
Lithium
Atomic# 4
Mass# 9.012
Beryllium
Atomic# 5
Mass# 10.811
Boron
Atomic# 6
Mass# 12.011
Carbon
Atomic# 7
Mass# 14.007
Nitrogen
Atomic# 8
Mass# 15.999
Oxygen
Atomic# 9
Mass# 18.998
Fluorine
Atomic# 10
Mass# 20.180
Neon
Atomic# 11
Mass# 22.990
Sodium
Atomic# 12
Mass# 24.305
Magnesium
Atomic# 13
Mass# 26.982
Aluminum
Si= Silicon
Atomic# 14
Mass# 28.086
P= Phosphorus
Atomic# 15
Mass# 30.974
S= Sulfur
Atmoic# 16
Mass# 32.066
Cl= Chlorine
Atomic# 17
Mass# 35.453
Ar= Argon
Atomic# 18
Mass# 39.948
K= Potassium
Atomic# 19
Mass# 39.098
Ca= Calcium
Atomic# 20
Mass# 40.078