2.1.2 h-j - LIPIDS
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
h) State the elements present in lipids.
carbon
hydrogen
oxygen (little)
h) State some characteristics of lipids.
Little oxygen present means that there are less polar bonds
hydrophobic
insoluble in water
soluble in organic solvents and ethanol
h) Define the term ‘macromolecule’.
Large complex molecules which are built from repeating units (monomers) with a large molecular weight.
h) State the additional element needed to make phospholipids.
phosphorus
h) State 3 categories of lipids.
Simple - triglycerides
Simple - steroids
Complex - phospholipid
h) Draw a labelled diagram showing the basic structure of triglyceride.
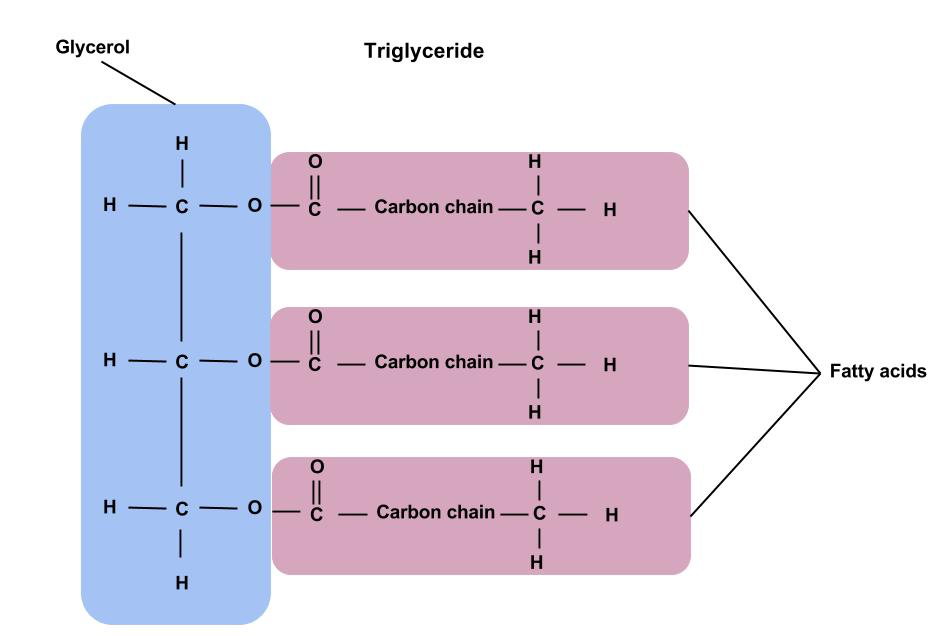
h) State the components of a triglyceride.
1 glycerol
3 fatty acids
h) Draw a diagram to show the difference between saturated, monounsaturated and polyunsaturated fatty acids/ triglycerides.
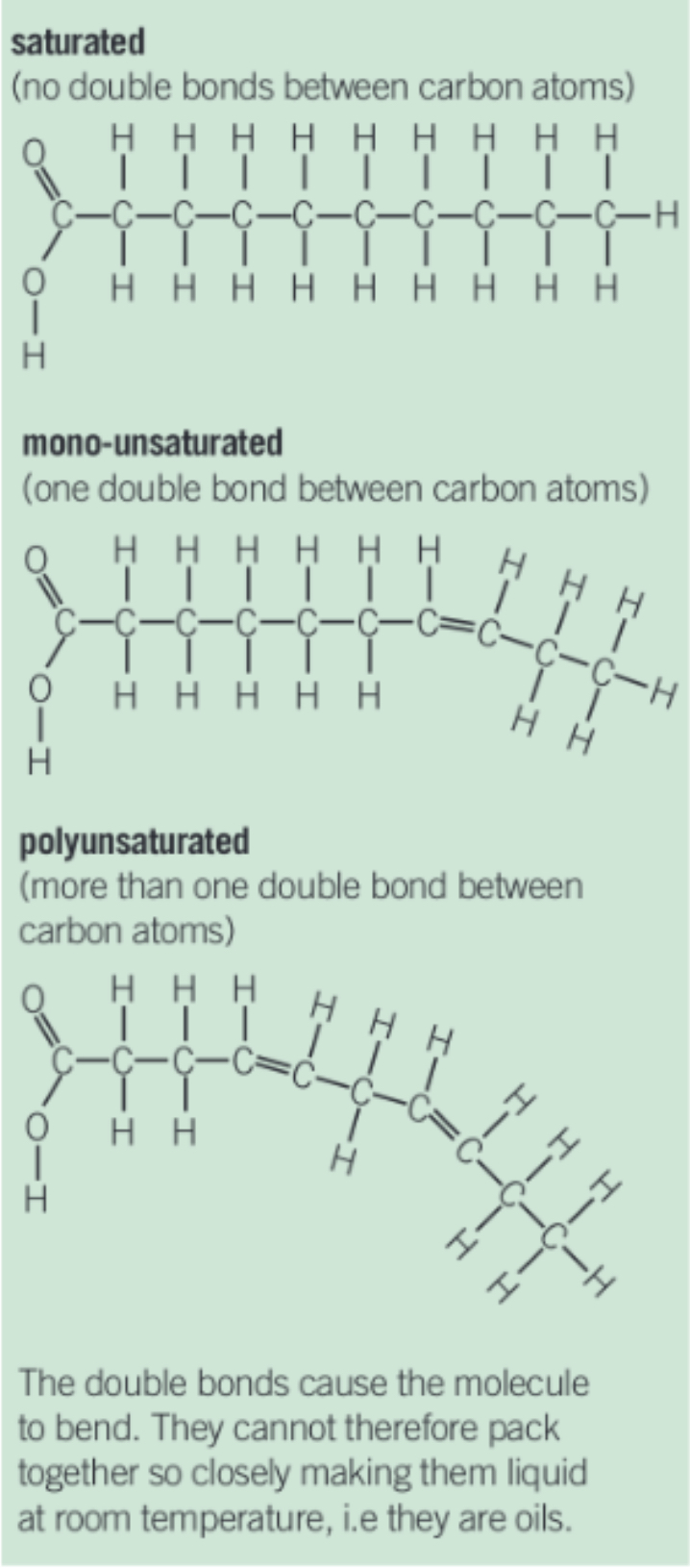
h) Explain why saturated triglycerides tend to be solid (fats) whereas unsaturated triglycerides tend to be liquid (oils) at room temperature.
This is because saturated triglycerides means that all carbons are linked to as many hydrogens as possible and can form many intermolecular bonds between molecules.
This forms straight chains, and is a fixed structure so it is a solid and has a higher melting point.
Unsaturated triglycerides have carbon-carbon double bonds which forms bent and kinked chains and have fewer intermolecular bonds between molecules.
This means that they cannot pack as closely together and therefore is liquid and has a lower melting point.
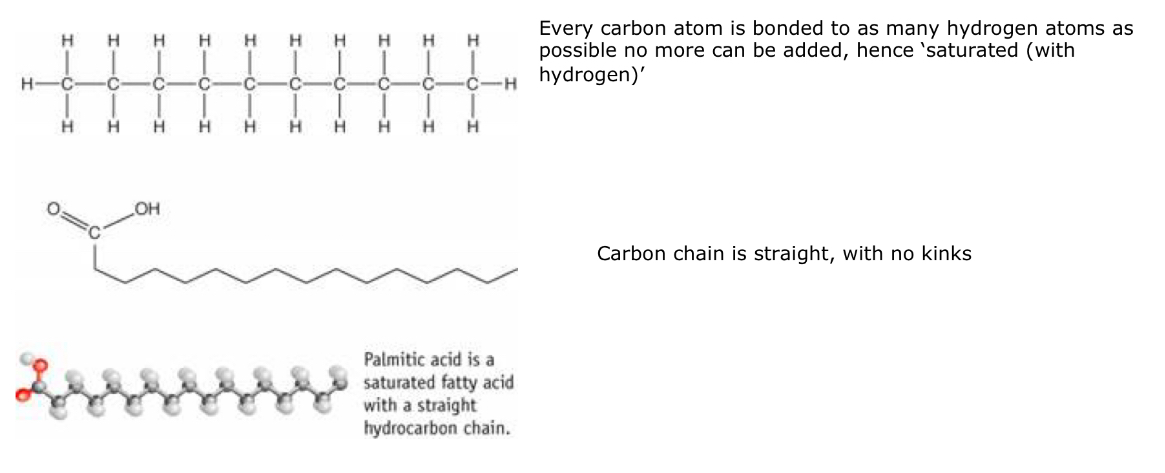
h) Describe and explain how the melting point of of triglyceride would correlate with the length of the fatty acids it contained.
As the length of the fatty acid chains increase, the melting point increases.
This is because longer hydrocarbon chains have more surface area for London forces, which increase intermolecular forces and in return require more energy to break them.
h) State the difference between the triglycerides found in non-fish animals and those in plants and fish.
Non-fish animals typically have triglycerides which are solid at room temperature because they have saturated fatty acids while those from plants and fish have triglycerides which are liquid due to a higher proportion of unsaturated fatty acid chains.
h) Draw a labelled diagram showing the basic structure of a phospholipid.
One fatty acid (hydrophobic) is replaced by a phosphate group (hydrophilic)
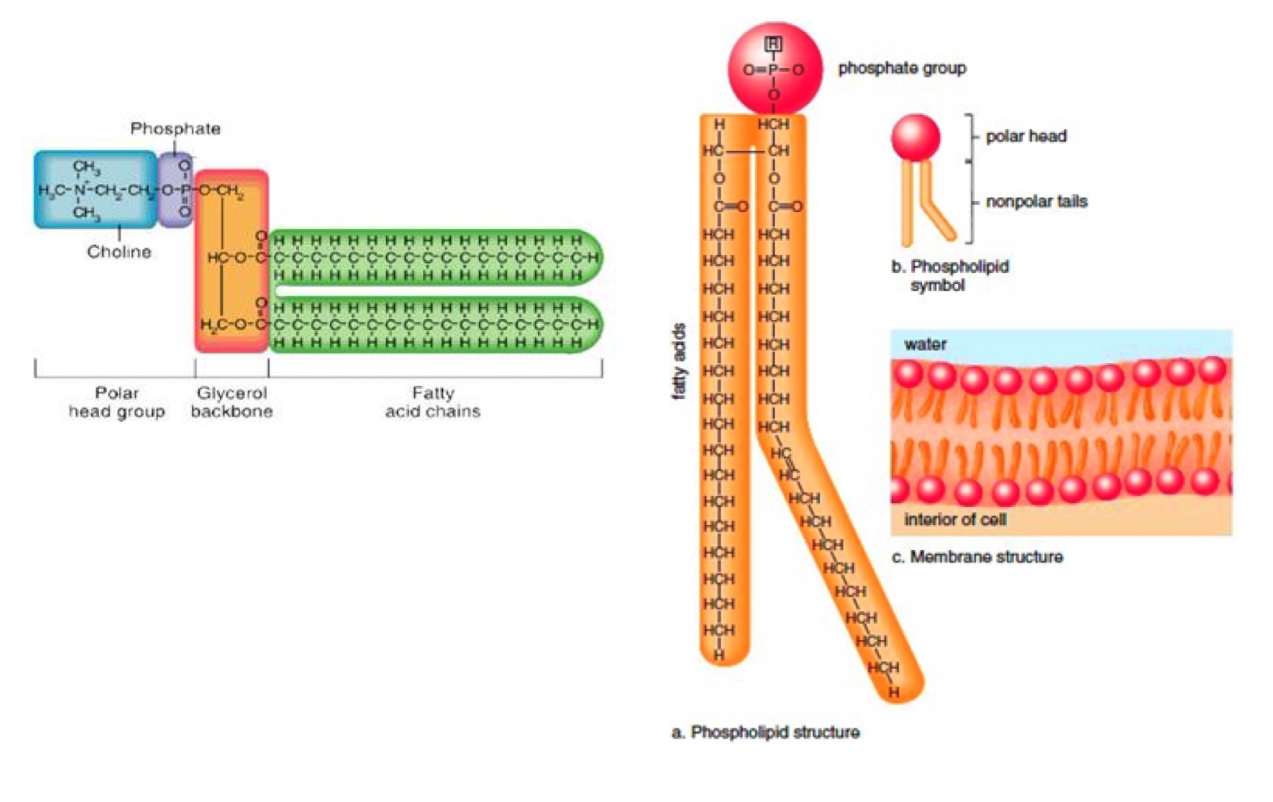
h) State the property that each end of the phospholipid has.
Polar head - hydrophilic
Non-polar tail - hydrophobic
h) Describe the difference between a triglyceride and a phospholipid.
A triglyceride has 3 fatty acids while a phospholipid has 2 fatty acids and a phosphate group.
A triglyceride is therefore non-polar, hydrophobic, making them suitable for energy storage, while a phospholipid is polar, with a hydrophilic head and hydrophobic tail, allowing them to form cell membranes.
i) Draw a diagram to show how a triglyceride is formed and broken down.
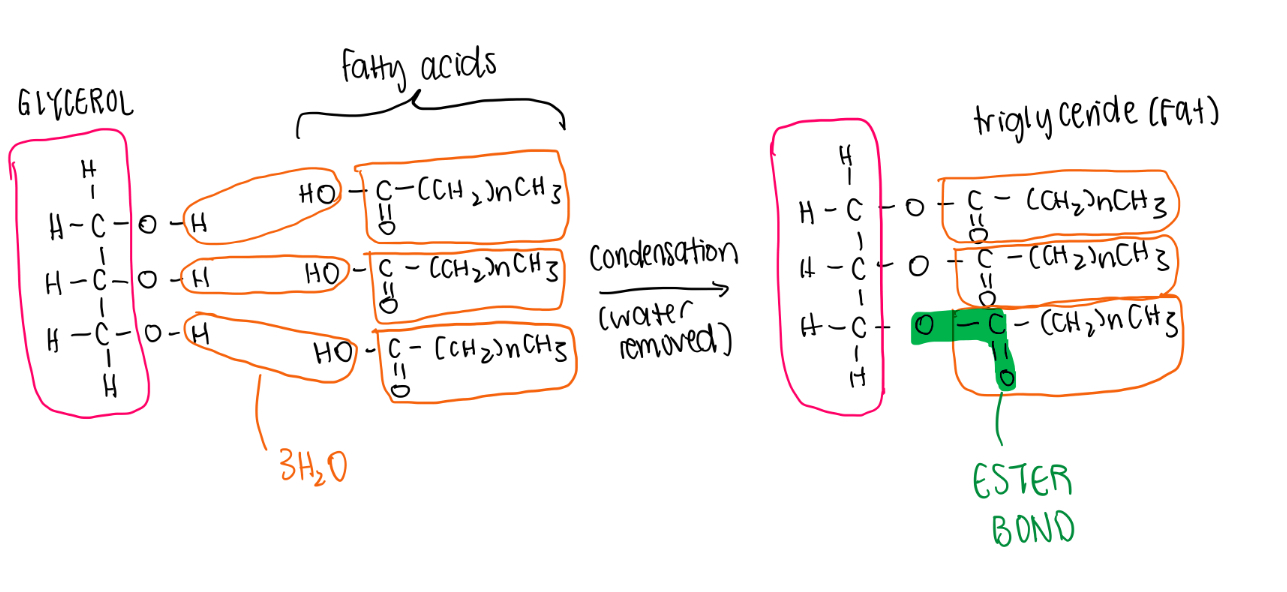
i) Name the bond that holds the building blocks of a triglyceride together.
ester bond
i) State the names of the reaction that forms triglycerides and the reaction that breaks them apart.
Forms - condensation reaction
Breaks - hydrolysis reaction
i) Explain how an ester bond is formed between glycerol and a fatty acid.
The hydroxyl groups from the glycerol and the fatty acids interact, leading to the formation of 3 water molecules and bonds between the fatty acids and the glycerol molecule.
i) State the number of water molecules produced in the production of one triglyceride.
3
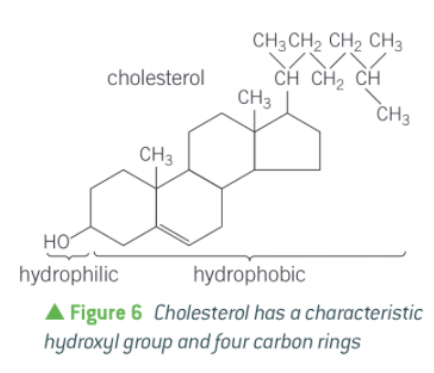
j) Describe the structure of sterols/ cholesterol.
They are complex alcohol molecules, based on a four carbon ring structure with a hydroxyl group (OH) at one end.
They are small and narrow which allows it to sit between phospholipids in membranes.
j) List the functions of triglycerides.
thermal insulation to reduce heat loss
cushioning to protect vital organs
electrical insulation necessary for impulse transmission (myelin sheath)
buoyancy for aquatic animals like whales
long term energy store
j) List the functions of phospholipid.
cell membrane formation
surfactants (surface active agents)
j) List the functions of cholesterol.
manufacturing of vitamin D
produces steroid hormones
produces bile for digestion
j) Explain why triglycerides store more energy per gram than carbohydrates.
This is their hydrocarbon chains have more C-H and C-C bonds, which store more chemical energy than C-O and O-H bonds found in carbohydrates.
j) Explain how phospholipid form a membrane.
The hydrophobic tails (fatty acids) orient themselves away from water as they are hydrophobic while the polar heads (phosphate group) orient themselves towards water because they are attracted to water and are hydrophilic.
In doing so, phospholipids arrange themselves into a bilayer structure which forms the basic structure of a cell membrane.
j) Describe how the presence of cholesterol affects the properties of cell membranes.
It is positioned between phospholipids with the hydroxyl group at the periphery of the membrane.
This adds stability to cell membranes and regulates their fluidity by keeping membranes fluid at low temps and stopping them becoming too fluid at high temps.