alkanes
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Alkanes
-saturated hydrocarbons, single carbon carbon bonds
-CnH2n+n
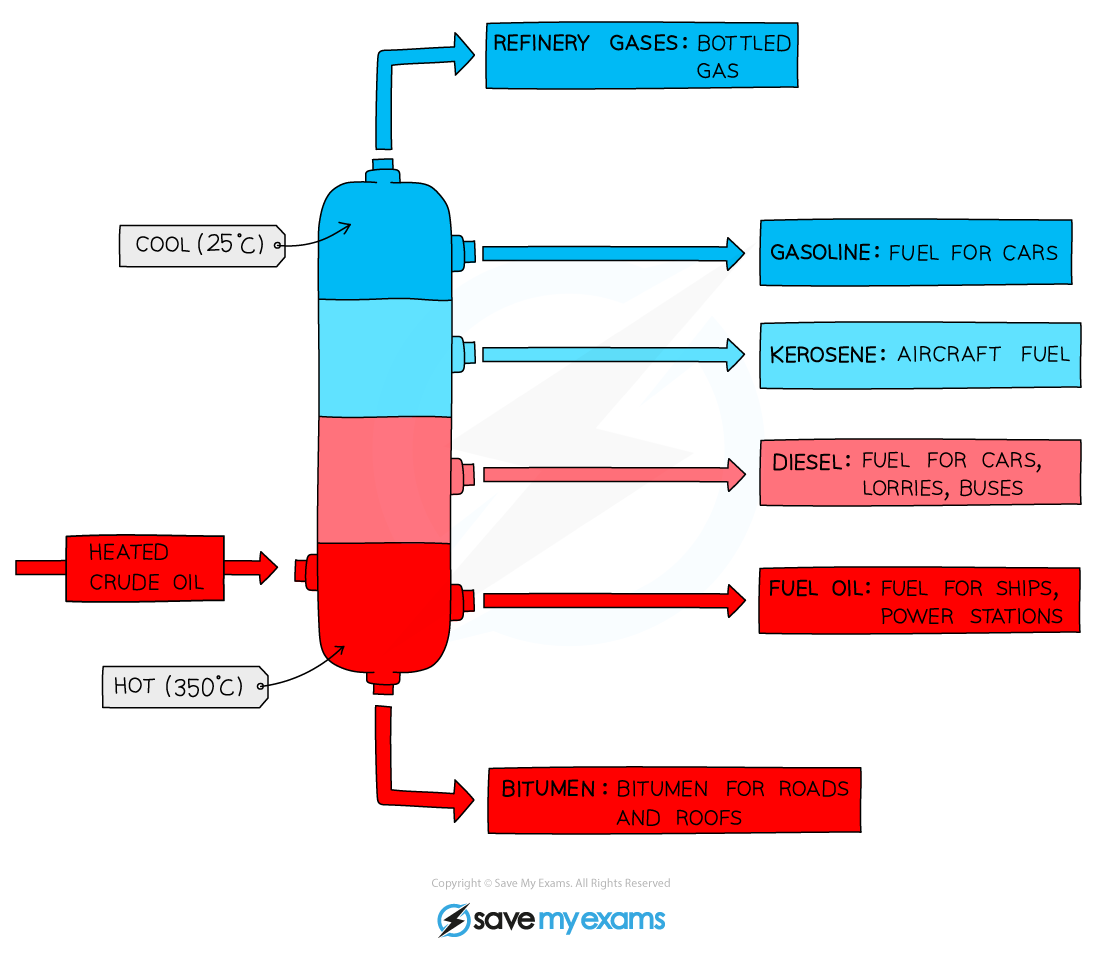
Fractional distillation
-crude oil- mixture of hydrocarbons
-extracted and transported to oil refinery
-products with short carbon chains have lower boiling points so rise up higher the column and are collected a the top
-can be broken down further with cracking
Reforming
-where alkanes are converted into chain lanes and cyclic hydrocarbons as they combust more efficiently
-often uses platinum catalyst
Cracking
-long carbon chains are broken down into smaller more useful ones
-require harsh conditions
Thermal cracking
-produces a high proportion of alkanes and alkanes
-high temp around 1200L and pressure around 7000kPa
-form alkanes and alkanes
Catalytic cracking
-produces aromatic compounds
-lower temperature with normal pressure and zeolite catalyst
Incomplete combustion
-car engine due to lack of o2
-form soot and include toxic cases like co, no and volatile organic compounds
Catalytic converters
-precious metals coated on a honeycomb to provide a large surface area
-2CO + O2 — 2CO2
2CO + 2NO — 2CO2 + N2
-nitrogen oxide is reduced on surface of hot catalyst
-not good at removing sulfur- should be removed before fuel is burned
Alternative fuels
-renewable
-biodiesel- made by refining renewable fats and oils
-bioethanol- fermentation
-biogas- made when organic waste breaks down
Benefits of biofuels
-carbon neutral
-as plats grow they absorb co2
-reduce amount of waste going to landfill as the waste can be used to produce them
-provide money for less developed countries as they have space to grow required crops
Drawbacks of biofuels
-expensive
-many developed countries don’t have space to make enough plats to make biofuels