free radical substitution (+ intro to mechanisms)
1/13
Earn XP
Description and Tags
ew
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
what is a haloalkane?
an alkane where at least 1 H atom has been replaced w/ a halogen atom
what is the product of free rad sub?
haloalkanes
what is a mechanism?
process showing the step by step movement of e- as reactants are converted into products
what does a single headed curly arrow represent?
movement of a single e-

what does a double headed curly arrow represent?
movement of an e- pair
what is a free radical?
a molecule w/ an odd no. of e- and so an unpaired e-
how reactive are free radicals?
very reactive!
name the steps of free rad sub:
initiation
propagation
termination
give the overall eqn for the reaction of methane w/ chlorine:
CH4 + Cl2 → CH3Cl + HCl
describe and give the eqn for the initiation step of free rad sub of the reaction of methane w/ chlorine:
UV light breaks Cl molecules into free radicals by providing the E to break the Cl-Cl bond
Cl : Cl → . Cl + . Cl
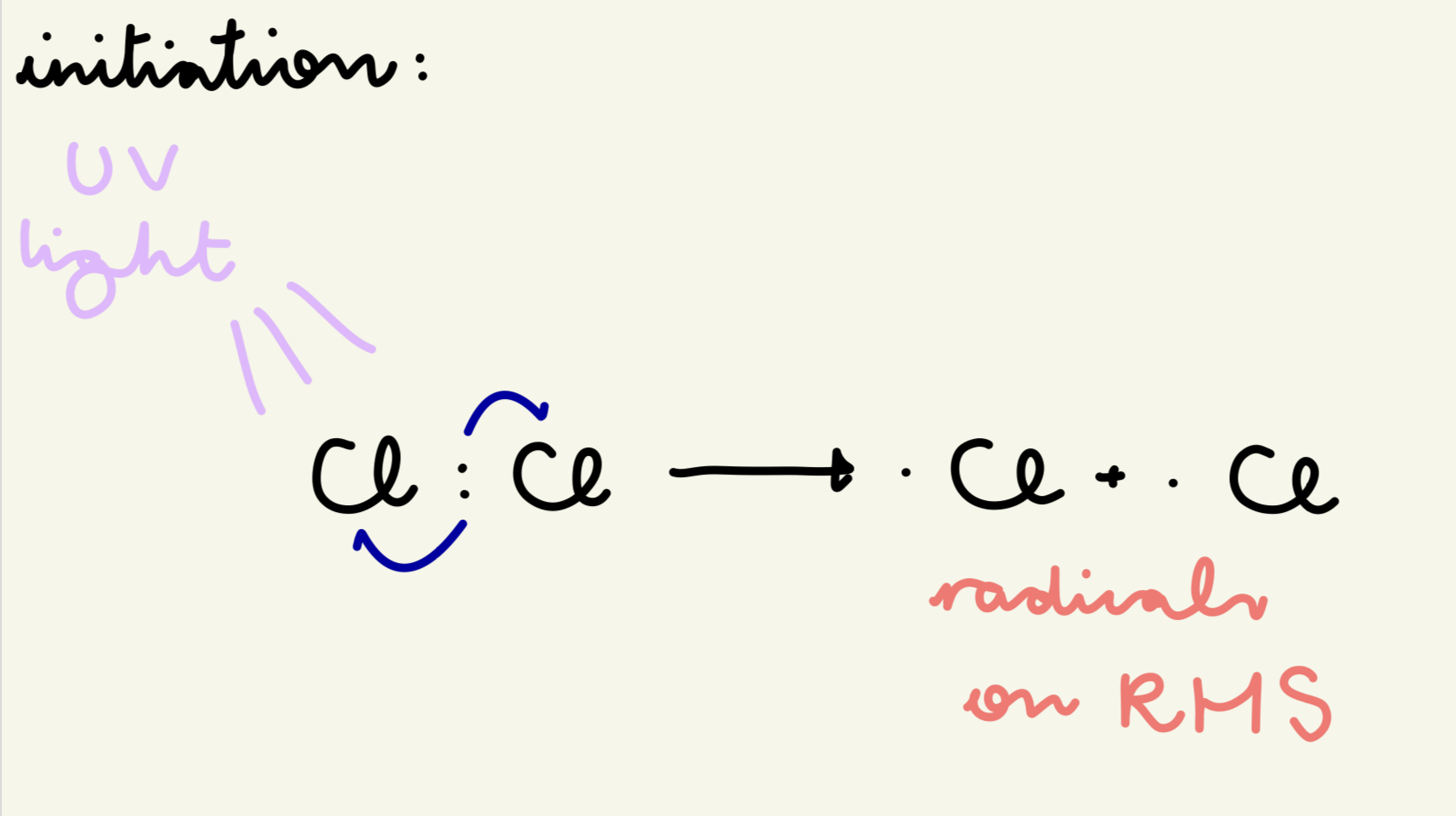
which side are the radicals on in initiation?
RHS
describe and give the eqn for the propagation step of free rad sub of the reaction of methane w/ chlorine:
Cl radicals react w/ alkanes in a chain reaction where the Cl radical acts as a catalyst
for each H replaced, there is a pair of propagation steps
. Cl + CH4 → HCl + . CH3
. CH3 + Cl2 → CH3Cl + . Cl
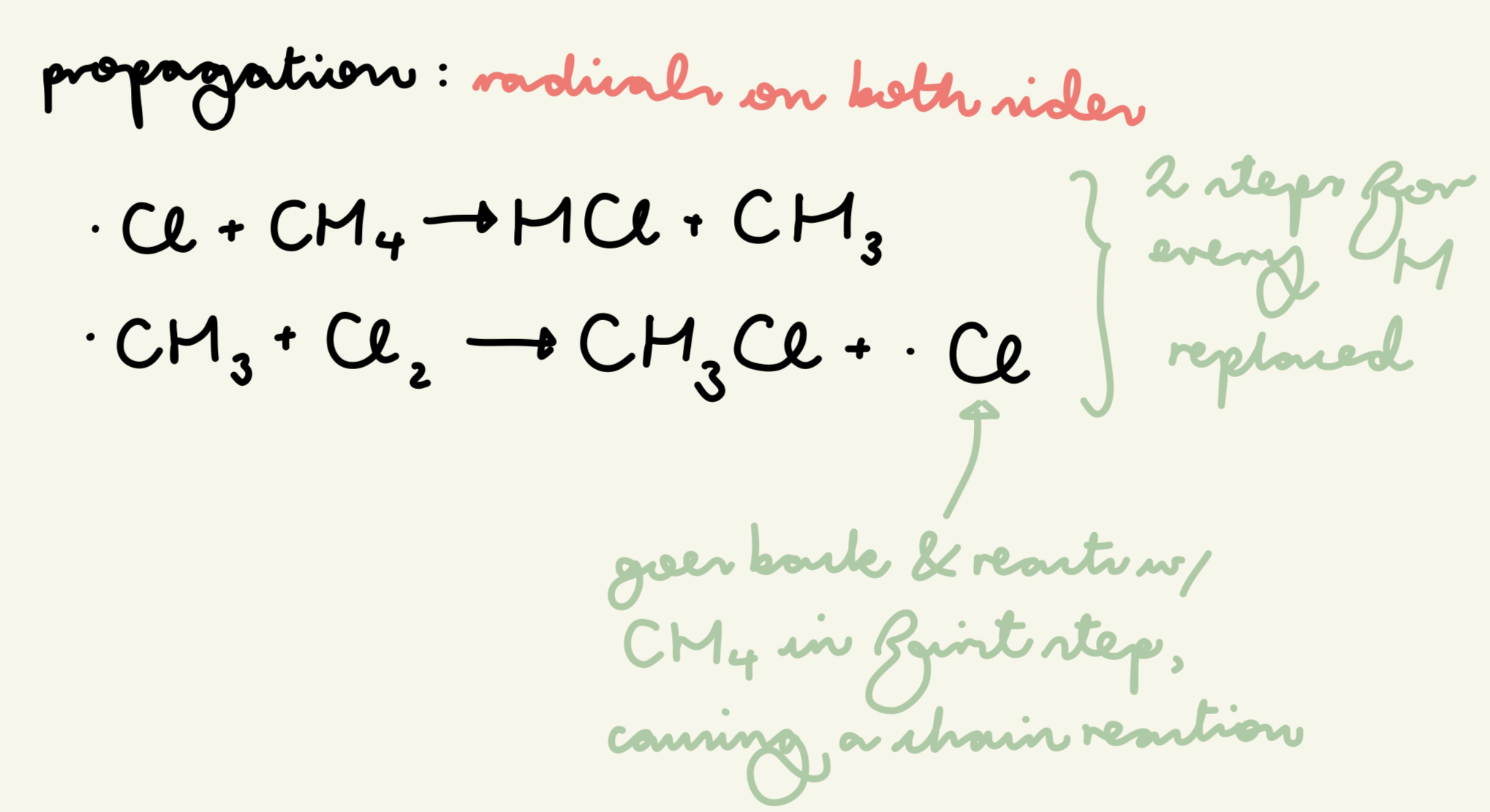
which side are the radicals on in propagation?
both sides
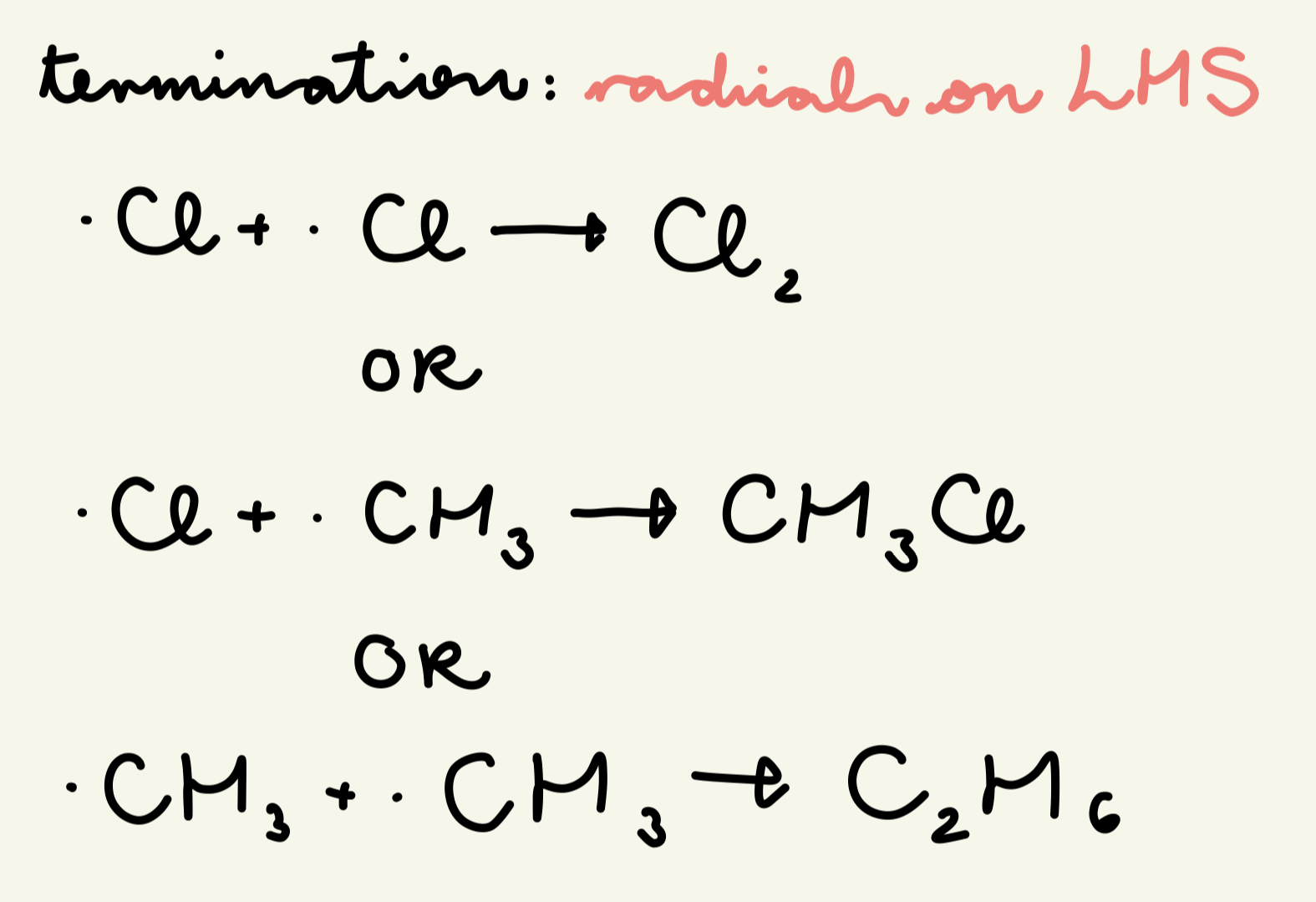
describe and give the eqns for the termination step of free rad sub of the reaction of methane w/ chlorine:
when any 2 free radicals meet, they react to form a molecule w/ no unpaired e-, stopping the chain reaction
. Cl + .Cl → Cl2
OR . Cl + . CH3 → CH3Cl
OR . CH3 + . CH3 → C2H6